154590-66-6
 154590-66-6 结构式
154590-66-6 结构式
基本信息
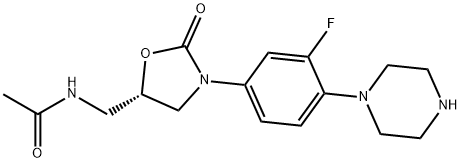
(S)-N-((3-(3-氟-4-(哌嗪-1-基)苯基)-2-氧噁唑啉丁-5-基)甲基)乙酰胺
N-[[(5S)-3-[3-氟-4-(1-哌嗪基)苯基]-2-氧代-5-恶唑烷基]甲基]乙酰胺
N-[[(5S)-3-[3-Fluoro-4-(1-piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]acetamide
Acetamide,N-[[(5S)-3-[3-fluoro-4-(1-piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]-
物理化学性质
制备方法
![1-Piperazinecarboxylic acid, 4-[4-[(5S)-5-[(acetylamino)methyl]-2-oxo-3-oxazolidinyl]-2-fluorophenyl]-, phenylmethyl ester](/CAS/20210111/GIF/174649-06-0.gif)
174649-06-0

154590-66-6
以化合物(CAS:174649-06-0)为原料合成(S)-N-((3-(3-氟-4-哌嗪-1-基苯基)-2-氧代噁唑烷-5-基)甲基)-乙酰胺的一般步骤如下:将0.4 g(0.85 mmol)的化合物15和0.06 g 10%钯/碳催化剂悬浮于19 mL甲醇和6 mL二氯甲烷的混合溶剂中,在氢气氛围下搅拌反应48小时。反应完成后,将反应混合物通过硅藻土垫过滤以去除催化剂。用50 mL二氯甲烷洗涤滤饼,随后用25 mL乙酸乙酯洗涤。合并滤液并浓缩,得到灰白色固体产物。将该固体产物用50 mL 10%甲醇-乙酸乙酯混合溶剂在温水浴中研磨30分钟,随后冷却至0℃以促进结晶。过滤收集结晶,得到化合物16,为白色固体。产率为91%;熔点为198-200℃;IR(KBr)υmax:3436和3329(NH),1741(C=O)和1644(CONH)cm-1;1H NMR(DMSO-d6)δ:9.03(br s,2H,NH2+),8.26(t,1H,J=6 Hz,CONH),7.50(dd,1H,J=15和2.5 Hz,H2氟苯),7.19(dd,1H,J=9和2.5 Hz,H6氟苯),7.12(t,1H,J=9 Hz,H5氟苯),4.71-4.68(m,1H,H5恶唑烷酮),4.07(t,1H,J=9 Hz,CH2NH),3.69(dd,1H,J=9和6 Hz,CH2NH),3.39(t,2H,J=6 Hz,H4恶唑烷酮),3.22-3.21(m,4H,哌嗪),3.17-3.16(m,4H,哌嗪),1.81(s,3H,CH3CO);MS:m/z 337(45,M+),295(100),250(53),191(32),57(32);元素分析计算值(C17H24ClFN4O3):C,52.78;H,6.25;Cl,9.16;F,4.91;N,14.48。实测值:C,52.94;H,6.10;Cl,9.24;F,4.73;N,14.60。
参考文献:
[1] Tetrahedron Letters, 2006, vol. 47, # 38, p. 6799 - 6802
[2] European Journal of Medicinal Chemistry, 2011, vol. 46, # 1, p. 65 - 70
[3] European Journal of Medicinal Chemistry, 2011, vol. 46, # 3, p. 893 - 900
[4] Patent: WO2011/37731, 2011, A1. Location in patent: Page/Page column 118-119
[5] Tetrahedron Letters, 1996, vol. 37, # 44, p. 7937 - 7940
