157327-42-9
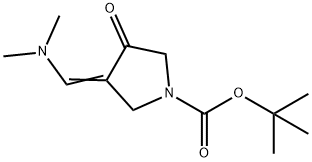 157327-42-9 结构式
157327-42-9 结构式
基本信息
3-((二甲氨基)亚甲基)-4-羰基吡咯烷-1-甲酸叔丁基酯
3-((二甲基氨基)亚甲基)-4-氧代吡咯烷-1-羧酸叔丁酯
叔-丁基 3-((二甲氨基)亚甲基)-4-羰基吡咯烷-1-羧酸酯
3-((dimethylamino)methylene)-4-oxopyrrolidine-1-carboxylate
tert-butyl 3-((dimethylamino)methylene)-4-oxopyrrolidine-1-c...
tert-Butyl 3-((diMethylaMino)Methylene)-4-oxopyrrolidine-1-carboxylate
(E)-TERT-BUTYL 3-((DIMETHYLAMINO)METHYLENE)-4-OXOPYRROLIDINE-1-CARBOXYLATE
tert-butyl (Z)-3-((dimethylamino)methylene)-4-oxopyrrolidine-1-carboxylate
3-DiMethylaMinoMethylene-4-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester
3-(diMethylaMinoMethylidene)-4-oxo-1-pyrrolidinecarboxylic acid tert-butyl ester
1-Pyrrolidinecarboxylic acid, 3-[(dimethylamino)methylene]-4-oxo-, 1,1-dimethylethyl ester
物理化学性质
制备方法
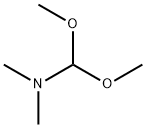
4637-24-5

101385-93-7

157327-42-9
步骤1:3-((二甲基氨基)亚甲基)-4-氧代吡咯烷-1-羧酸叔丁酯的合成。将1-叔丁氧碳基-3-吡咯烷酮(10g,54.05mmol)悬浮于N,N-二甲基甲酰胺二甲基缩醛(120mL)中,在105℃下搅拌反应1小时。反应完成后,将反应混合物冷却至0℃。随后,在室温下通过减压蒸馏除去溶剂。残余物通过硅胶柱色谱法(洗脱剂:二氯甲烷/甲醇=60/1至20/1)进行纯化,得到目标产物3-((二甲基氨基)亚甲基)-4-氧代吡咯烷-1-羧酸叔丁酯(10.78g,收率83%)。产物经ESI-LCMS分析显示m/z:241.2 [M+1]+;1H NMR(400MHz,CDCl3)δppm:7.26(s,1H),4.57(s,2H),3.86(s,2H),3.09(s,6H),1.48(s,9H)。
参考文献:
[1] Patent: WO2016/44641, 2016, A2. Location in patent: Paragraph 00377
[2] Heterocycles, 2002, vol. 56, # 1-2, p. 257 - 264
[3] Patent: US2013/237538, 2013, A1. Location in patent: Paragraph 0308
[4] Patent: US2013/237537, 2013, A1. Location in patent: Paragraph 0292-0293
[5] Patent: WO2007/97931, 2007, A2. Location in patent: Page/Page column 32
