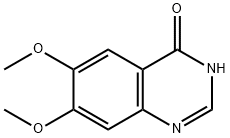
16064-15-6
 16064-15-6 结构式
16064-15-6 结构式
基本信息
埃罗替尼杂质78
埃罗替尼杂质75
厄洛替尼杂质A55
厄洛替尼杂质EL-5-110
6,7-二羟基喹唑啉-4-酮
6,7-二羟基喹唑啉-4(3H)-酮
Erlotinib Impurity 84
6,7-Dihydroxyquinazolin-4-one
7-dihydroxyquinazolin-4(3H)-one
6,7-dihydroxy-3H-quinazolin-4-one
6,7-dihydroxyquinazolin-4(3H)-one
4(3H)-Quinazolinone, 6,7-dihydroxy-
6,7-Dihydroxy-3,4-dihydroquinazolin-4-one
6,7-dihydroxyquinazolin-4(3H)-one EL-7-107
物理化学性质
制备方法
13794-72-4

16064-15-6
以6,7-二甲氧基喹唑啉-4-酮为原料合成6,7-二羟基喹唑啉-4(3H)-酮的一般步骤: (i)在配备机械搅拌器、回流冷凝器和温度计的2.0L四颈圆底烧瓶中,加入48%(w/w)氢溴酸(1000g)和6,7-二甲氧基-4(3H)-喹唑啉酮(100g)。缓慢加热反应混合物至110℃,并在此温度下保持1小时。随后升温至回流条件,回流12小时。反应进程通过TLC监控。反应完成后,冷却反应混合物至25-35℃,过滤。将湿滤饼转移至另一个含有1000ml去离子水的2.0L圆底烧瓶中,搅拌10-15分钟,并通过加入氨水溶液调节pH至7.0-7.5。过滤所得产物,用去离子水洗涤滤饼,干燥后得到85.2g(理论产率98.62%)6,7-二羟基-4(3H)-喹唑啉酮,为灰白色结晶固体。产物纯度:99.25%(HPLC);熔点:>250℃。IR(KBr,cm-1):3208.7, 1679.0, 1614.5, 1514.7, 1427.7, 1374.3, 1316.2, 1293.9, 1261.0, 1214.5, 1195.5, 866.0, 845.3, 780.7, 523.8, 449.2。1H NMR(300MHz,DMSO-d6):δ 6.93(s, 1H), 7.35(s, 1H), 7.84(s, 1H), 9.75(s, 1H), 10.13(s, 1H), 11.2-12.4(s, 1H)。MS:179(M+1),177(M-1)。
参考文献:
[1] Patent: US2009/306377, 2009, A1. Location in patent: Page/Page column 5-6
[2] Patent: WO2016/123706, 2016, A1. Location in patent: Paragraph 00170
[3] Patent: WO2008/76949, 2008, A2. Location in patent: Page/Page column 11; 29
[4] Patent: WO2009/121042, 2009, A1. Location in patent: Page/Page column 39
[5] Patent: CN108727400, 2018, A. Location in patent: Paragraph 0055; 0057; 0059