16694-17-0
 16694-17-0 结构式
16694-17-0 结构式
基本信息
4-溴-3-噻吩甲酸
4-溴噻吩-3-甲酸
4-溴噻吩-3-羧酸
4-bromo-3-thenoic acid
4-Bromo-3-carboxythiophene
4-Bromothiophenecarboxylicacid
4-Bromo-3-thiophenecarboxylicacid
4-Bromothiophene-3-carvoxylic acid
4-BROMOTHIOPHENE-3-CARBOXYLIC ACID
3-Bromo-4-thiophenecarboxylic acid
3-Thiophenecarboxylic acid, 4-broMo-
4-Bromo-3-thiophenecarboxylic acid 97%
物理化学性质
安全数据
制备方法

3141-26-2
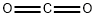
124-38-9

16694-17-0
在氮气氛围下,将100 mL乙醚冷却至-78℃。缓慢加入1.6 M正丁基锂溶液(28.4 mL)。随后,在10分钟内滴加3,4-二溴噻吩(10 g,41.3 mmol)溶解于50 mL乙醚的溶液。维持-78℃反应温度,继续搅拌10分钟后,加入过量(>50 g)新鲜粉末状干冰(CO?)。反应混合物在-78℃下搅拌1小时,然后缓慢加入30 mL 1 M NaOH溶液(预先用100 mL水稀释)(注意:CO?会释放)。逐渐升温至冰融化。分离有机相和水相,用25 mL 1 N NaOH溶液萃取乙醚相。合并所有水相,用100 mL 1 N HCl溶液酸化至pH<7。过滤收集析出的白色沉淀,用水洗涤,并在真空烘箱中干燥,得到4-溴噻吩-3-羧酸白色固体(5.8 g,收率68%)。
参考文献:
[1] Pharmazie, 2006, vol. 61, # 11, p. 901 - 907
[2] Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1994, # 19, p. 2735 - 2744
[3] Patent: WO2006/3096, 2006, A1. Location in patent: Page/Page column 71-72
[4] Patent: US2013/116233, 2013, A1. Location in patent: Paragraph 1018; 1019
[5] Patent: WO2014/140086, 2014, A1. Location in patent: Page/Page column 68-69
