17168-45-5
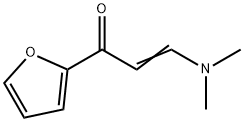 17168-45-5 结构式
17168-45-5 结构式
基本信息
(E)-3-(dimethylamino)-1-(furan-2-yl)prop-2-en-1-on
(E)-3-(dimethylamino)-1-(furan-2-yl)prop-2-en-1-one
物理化学性质
制备方法

1192-62-7
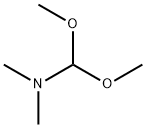
4637-24-5

17168-45-5
一般步骤:将2-乙酰基呋喃(25.0 g,0.227 mol)与N,N-二甲基甲酰胺二甲基缩醛(40 mL)混合,在100℃下搅拌反应9小时。反应完成后,将混合物冷却至室温,随后进行浓缩以去除挥发性组分。向浓缩后的残余物中加入乙醚和己烷,通过过滤收集析出的固体产物。用己烷洗涤固体,最终得到3-(二甲氨基)-1-(呋喃-2-基)丙-2-烯-1-酮(36.5 g,收率97%),为棕色固体。产物经1H NMR(400 MHz,DMSO-d6)表征,化学位移δ(ppm)如下:2.88(3H,宽峰),3.14(3H,宽峰),5.65(1H,双峰,J = 12.6 Hz),6.60(1H,双双重峰,J = 2.0, 3.4 Hz),7.10(1H,双双重峰,J = 0.8, 3.4 Hz),7.68(1H,双峰,J = 12.6 Hz),7.79(1H,双双重峰,J = 0.8, 2.0 Hz)。
参考文献:
[1] Patent: EP1439175, 2004, A1. Location in patent: Page 45-46
[2] Patent: US2004/6082, 2004, A1. Location in patent: Page/Page column 22
[3] Journal of Heterocyclic Chemistry, 1996, vol. 33, # 6, p. 1707 - 1710
[4] Organic Process Research and Development, 2008, vol. 12, # 3, p. 490 - 495
[5] Journal of the Korean Chemical Society, 2011, vol. 55, # 2, p. 243 - 250
