17720-60-4
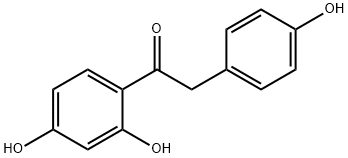 17720-60-4 结构式
17720-60-4 结构式
基本信息
1-(2,4-二羟基苯基)-2-(4-羟基苯基)乙酮
2',4'-二羟基-2-(4-羟基苯基)苯乙酰酮,97%
1-(2,4-二羟基苯基)-2-(4-羟基苯基)乙-1-酮
OTAVA-BB BB7020210418
2,4,4'-Trihydroxydeoxybenzoin
2,4-Dihydroxyphenyl 4'-hydroxybenzyl ketone
1-(2,4-DIHYDROXY-PHENYL)-2-(4-HYDROXY-PHENYL)-ETH
2',4'-Dihydroxy-2-(4-hydroxyphenyl)acetophenone,97%
Ethanone,1-(2,4-dihydroxyphenyl)-2-(4-hydroxyphenyl)-
1-(2,4-Dihydroxyphenyl)-2-2-(4-hydroxyphenyl)ethanone
1-(2,4-DIHYDROXYPHENYL)-2-(4-HYDROXYPHENYL)ETHAN-1-ONE
1-(2,4-DIHYDROXY-PHENYL)-2-(4-HYDROXY-PHENYL)-ETHANONE
物理化学性质
制备方法

156-38-7
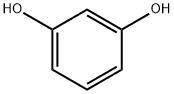
108-46-3

17720-60-4
步骤1:1-(2,4-二羟基苯基)-2-(4-羟基苯基)乙酮的合成 在氮气保护下,将间苯二酚(62.000 g, 563.1 mmol, 1.0当量)和4-羟基苯乙酸(94.237 g, 619.4 mmol, 1.1当量)加入装有机械搅拌桨、压力平衡加料漏斗、温度计及加热套的2 L三颈圆底烧瓶中。加入甲苯(350 mL)形成悬浮液。通过加料漏斗缓慢滴加三氟化硼醚合物(198.201 mL, 1578.0 mmol, 2.8当量),控制滴加速度为3-4 mL/min,同时以150 rpm搅拌反应混合物。反应过程中,内部温度逐渐升至100°C,溶液颜色由黄色逐渐变为深红色。滴加完毕后,移除加料漏斗,换装冷凝器,继续在108°C下搅拌反应1.5小时。通过HPLC分析确认反应完成。停止加热,冷却反应混合物至室温,静置分层。在搅拌下缓慢加入12%乙酸钠水溶液(41 g溶于336 mL水),继续搅拌16小时。析出的固体经过滤收集于烧结玻璃漏斗中,真空干燥16小时,得到白色粉末状产物(119.67 g, 产率87.0%)。
参考文献:
[1] Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1991, # 12, p. 3005 - 3008
[2] Journal of Organic Chemistry, 2000, vol. 65, # 8, p. 2305 - 2308
[3] Journal of Agricultural and Food Chemistry, 1994, vol. 42, # 9, p. 1869 - 1871
[4] Tetrahedron Letters, 2006, vol. 47, # 47, p. 8375 - 8378
[5] Patent: WO2013/90921, 2013, A1. Location in patent: Paragraph 00165
