187022-49-7
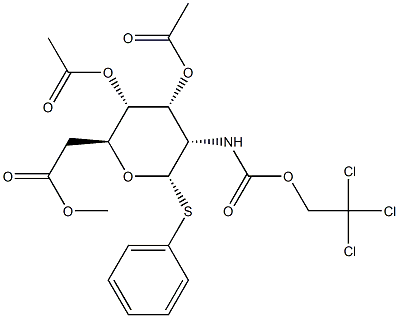 187022-49-7 结构式
187022-49-7 结构式
基本信息
苯基3,4,6-三-O-乙酰基-2-脱氧-1-硫代-2-(2,2,2-三氯乙氧基甲酰氨基)-Β-D-吡喃葡萄糖苷
苯基 3,4,6-三-O-乙酰基-2-脱氧-1-硫代-2-(2,2,2-三氯乙氧基甲酰胺基)-Β-D-吡喃葡萄糖苷
苯基 2-脱氧-1-硫代-2-[[(2,2,2-三氯乙氧基)羰基]氨基]-Β-D-吡喃葡萄糖苷3,4,6-三乙酸酯
苯基 2-脱氧-1-硫代-2-[[(2,2,2-三氯乙氧基)羰基]氨基]-Β-D-吡喃葡萄糖苷3,4,6-三乙酸酯
苯基 3,4,6-三-O-乙酰基-2-脱氧-1-硫代-2-(2,2,2-三氯乙氧基甲酰胺基)-Β-D-吡喃葡萄糖苷
苯基-3,4,6-三-O-乙酰基-2-脱氧-1-硫代-2-(2,2,2-三氯乙氧基甲酰氨基)-BETA-D-吡喃葡萄糖苷
(2R,3S,4R,5R,6S)-2-(乙酰氧基甲基)-6-(苯硫基)-5-(((2,2,2-三氯乙氧基)羰基)氨基)四氢-2H-吡喃-3,4-二基二乙酸酯
苯基-3,4,6-三-O-乙酰基-2-脱氧-1-硫代-2-(2,2,2-三氯乙氧基甲酰氨基)- Β-D-吡喃葡萄糖苷
PHENYL 3,4,6-TRI-O-ACETYL-2-DEOXY-1-THIO-2-(2,2,2-?TRICHLOROETHOXYFORMA?MIDO)-Β-D-GLUCOPYRANOSIDE苯基 3,4,6-三-O-乙酰基-2-脱氧-1-硫代-2-(2,2,2-三氯乙氧基甲酰胺基)-Β-D-吡喃葡萄糖苷
Phenyl 3,4,6-Tri-O-acetyl-2-deoxy-1-thio-2-(2,2,2-trichloroethoxyforMaMido)-β-D-glucopyranoside
Phenyl 3,4,6-Tri-O-acetyl-2-deoxy-1-thio-2-(2,2,2-trichloroethoxyformamido)-β-D-glucopyranoside
Phenyl 3,4,6-Tri-O-acetyl-2-deoxy-1-thio-2-(2,2,2-trichloroethoxyformamido)-β-D-galactopyranoside
Phenyl3,4,6-Tri-O-acetyl-2-deoxy-1-thio-2-(2,2,2-trichloroethoxyformamido)-β-D-glucopyranoside>
Phenyl 3,4,6-Tri-O-acetyl-2-deoxy-1-thio-2-(2,2,2-trichloroethoxyformamido)-beta-D-glucopyranoside
Phenyl 3,4,6-Tri-O-acetyl-2-deoxy-1-thio-2-(2,2,2-trichloroethoxycarbonylamino)-β-D-glucopyranoside
Phenyl 3,4,6-Tri-O-acetyl-2-deoxy-1-thio-2-(2,2,2-trichloroethoxycarbonylamino)-β-D-glucopyranoside
Phenyl 3,4,6-Tri-O-acetyl-2-deoxy-1-thio-2-(2,2,2-trichloroethoxyformamido)-b-D-glucopyranoside, 98%
Phenyl 2-deoxy-1-thio-2-[[(2,2,2-trichloroethoxy)carbonyl]amino]-β-D-glucopyranoside 3,4,6-triacetate
物理化学性质
制备方法
108-98-5
![α-D-Glucopyranose, 2-deoxy-2-[[(2,2,2-trichloroethoxy)carbonyl]amino]-, 1,3,4,6-tetraacetate](/CAS/20210305/GIF/122210-01-9.gif)
122210-01-9
![苯基 2-脱氧-1-硫代-2-[[(2,2,2-三氯乙氧基)羰基]氨基]-Β-D-吡喃葡萄糖苷3,4,6-三乙酸酯](/CAS/20180808/GIF/187022-49-7.gif)
187022-49-7
一般步骤:将D-葡糖胺盐酸盐(20 g,92.8 mmol)溶解于水(250 mL)中,加入饱和NaHCO3水溶液(250 mL)和氯甲酸2,2,2-三氯乙酯(14.05 mL,102 mmol),剧烈搅拌至室温。反应混合物通过烧结漏斗过滤,收集白色固体并真空干燥过夜。将所得固体溶于吡啶(100 mL)中,冷却至0℃,缓慢加入乙酸酐(100 mL),随后加入4-二甲氨基吡啶(DMAP,500 mg,4.1 mmol)。将反应液缓慢升温至室温并搅拌12小时。反应完成后,用二氯甲烷(200 mL)稀释反应液,依次用1M HCl(3×100 mL)、饱和NaHCO3水溶液(2×100 mL)和盐水(100 mL)洗涤,有机相用无水Na2SO4干燥,过滤后减压浓缩,得到目标产物(2R,3S,4R,5R,6S)-2-(乙酰氧基甲基)-6-(苯硫基)-5-(((2,2,2-三氯乙氧基)羰基)氨基)四氢-2H-吡喃-3,4-二基二乙酸酯(葡糖胺11),为白色泡沫状固体(44.3 g,产率91%)。产物的1H NMR谱(400 MHz,CDCl3)与文献报道一致[53],主要信号如下:δ 6.23 (1H, d, J = 3.4 Hz), 5.28 (1H, dd, J = 10.7, 9.6 Hz), 5.20 (1H, t, J = 9.8 Hz), 5.14 (1H, d, J = 9.3 Hz, NHTroc), 4.82 (1H, d, J = 12.1 Hz), 4.62 (1H, d, J = 12.1 Hz), 4.28 (1H, dd, J = 12.4, 4.0 Hz), 4.20 (1H, ddd, J = 10.7, 9.4, 3.8 Hz), 4.06 (1H, dd, J = 12.5, 2.4 Hz), 4.06-4.01 (1H, m), 2.20 (3H, s), 2.09 (3H, s), 2.04 (6H, s)。
参考文献:
[1] Chemical and Pharmaceutical Bulletin, 1997, vol. 45, # 2, p. 312 - 320
[2] Carbohydrate Research, 2017, vol. 452, p. 47 - 53
[3] Journal of Organic Chemistry, 2011, vol. 76, # 10, p. 3691 - 3709
[4] Angewandte Chemie - International Edition, 2014, vol. 53, # 31, p. 8190 - 8194
[5] Angew. Chem., 2014, vol. 126, # 31, p. 8329 - 8333,5
