18720-35-9
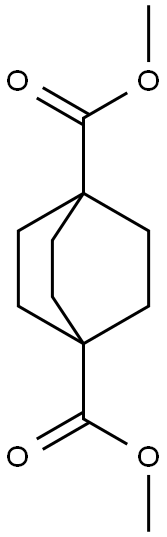
 18720-35-9 结构式
18720-35-9 结构式
基本信息
双环[2.2.2]辛烷-1,4-二羧酸单甲酯
4-(甲氧羰基)双环[2.2.2]辛烷-1-甲酸
4-(甲氧羰基)双环[2.2.2]辛烷-1-羧酸
二环【2,2,2】辛烷-1,4-环己二羧酸单甲酯
双环[2.2.2]辛烷-1,4-环己二羧酸单甲酯
4-(甲氧基羰基)双环[2.2.2]辛烷-1-甲酸
英文名称:BICYCLO[2.2.2]OCTANE-1,4-DICARBOXYLIC ACID HEMIMETHYL ESTER
4-(METHOXYCARBONYL)BICYCLO[2.2.2]OCTANE-1-CARBOXYLIC ACID 4-(甲氧羰基)双环[2.2.2]辛烷-1-甲酸CAS:18720-35-9
4-(Methoxycarbonyl)bicyclo[2.2.2]octane-1-
1-Carboxy-4-(Methoxycarbonyl)bicyclo[2.2.2]octane
Bicyclo [2.2.2] octane-1,4-cyclohexanedicarboxylate
4-(Methoxycarbonyl)bicyclo[2.2.2]octan-1-carboxylic Acid
4-(METHOXYCARBONYL)BICYCLO[2.2.2]OCTANE-1-CARBOXYLICACID
Monomethyl Hydrogen Bicyclo[2.2.2]octane-1,4-dicarboxylate
BICYCLO[2.2.2]OCTANE-1,4-DICARBOXYLIC ACID HEMIMETHYL ESTER
Bicyclo [2.2.2]octane-1,4-dicarboxylic acid, 1-methyl ester
Bicyclo[2.2.2]octane-1,4-dicarboxylIic acid hemimethyl este
物理化学性质
制备方法
1459-96-7

18720-35-9
以二环[2.2.2]辛烷-1,4-二甲酸二甲酯为原料合成4-(甲氧羰基)双环[2.2.2]辛烷-1-羧酸的一般步骤:将4-(甲氧羰基)双环[2.2.2]辛烷-1-羧酸(20g)与氢氧化钾(5.5g)在甲醇/水(10:1,106mL)中的混合物加热回流过夜。反应完成后,冷却至室温并浓缩。将残余物用乙酸乙酯稀释,并用1N氢氧化钠水溶液(2×100mL)萃取。有机相用硫酸镁干燥,过滤并浓缩,得到白色固体状的起始原料。合并的水相用2N盐酸水溶液调节至pH 1-2,并用乙酸乙酯(4×250mL)萃取。合并的混浊有机相通过槽纹过滤器过滤,用饱和氯化钠水溶液洗涤,硫酸镁干燥,过滤并浓缩,得到4-(甲氧羰基)双环[2.2.2]辛烷-1-羧酸,为无色固体(13.1g,收率70%)。质谱分析显示[MH]+ = 213。
参考文献:
[1] Bioorganic and Medicinal Chemistry Letters, 2014, vol. 24, # 24, p. 5731 - 5737
[2] Patent: WO2006/128184, 2006, A2. Location in patent: Page/Page column 164-165
[3] Patent: WO2008/63671, 2008, A2. Location in patent: Page/Page column 177-178
[4] Patent: WO2014/159802, 2014, A1. Location in patent: Paragraph 00189
[5] Journal of Medicinal Chemistry, 2009, vol. 52, # 6, p. 1558 - 1568
