189442-87-3
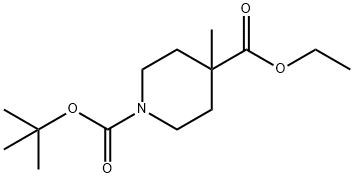 189442-87-3 结构式
189442-87-3 结构式
基本信息
N-叔丁氧羰基-4-甲基异哌啶甲酸乙酯
1-BOC-4-甲基-4-哌啶甲酸乙酯
N-BOC-4-甲基-4-哌啶甲酸乙酯
N-叔丁氧羰基-4-甲基-4-哌啶甲酸乙酯
N-BOC-4-METHYL-4-METHYLPIPERIDINECARBOXYLATE
N-BOC-4-METHYL ISONIPECOTIC ACID ETHYL ESTER
Ethyl 1-Boc-4-methylpiperidine-4-carboxylate
ETHYL N-BOC-4-METHYLPIPERIDINE-4-CARBOXYLATE
1-Boc-4-Methyl-isonipecotic acid ethyl ester
ETHYL 1-N-BOC-4-METHYL-PIPERIDINE-CARBOXYLATE
Ethyl N-Boc-4-methylpiperidine-4-carboxylate ,97%
N-Boc-4-Methyl isonipecotic acid ethyl ester ,95%
1,4-PIPERIDINEDICARBOXYLIC ACID, 4-1-(1,1-DIMETHYL
物理化学性质
安全数据
toxic compounds or compounds which causing chronic effects
Eye Dam. 1
Skin Irrit. 2
STOT SE 3
制备方法

142851-03-4
74-88-4

189442-87-3
将N-Boc-4-哌啶甲酸乙酯(10.00g,36.92mmol)溶解于四氢呋喃(THF,100mL)中,并将溶液冷却至-78℃。在氮气保护下,缓慢加入二异丙基氨基锂(LDA,47mmol,23mL),保持温度在-78℃,搅拌反应混合物1小时。随后,逐滴加入碘甲烷(81.25mmol,5.08mL),继续在-78℃下搅拌1小时。反应完成后,移除冷浴,使反应混合物缓慢升温至室温,并继续搅拌30分钟。用饱和氯化铵(NH4Cl)溶液淬灭反应,然后用乙酸乙酯(EtOAc)进行分配萃取。合并有机相,用无水硫酸镁(MgSO4)干燥,过滤后减压浓缩,得到目标产物N-Boc-4-甲基-4-哌啶甲酸乙酯,收率定量。产物经1H NMR(400MHz,DMSO-d6)表征,化学位移δ:4.11(q,J=7.1Hz,2H),3.61(dt,J=13.4Hz,J=4.5Hz,2H),2.95(d,J=0.3Hz,2H),1.91(d,J=13.6Hz,2H),1.39(s,9H),1.31(m,2H),1.19(m,3H),1.15(s,3H)。
参考文献:
[1] Patent: WO2015/86527, 2015, A1. Location in patent: Page/Page column 113; 114
[2] Patent: WO2015/86525, 2015, A1. Location in patent: Page/Page column 117
[3] Patent: WO2008/121687, 2008, A2. Location in patent: Page/Page column 43; 89; 94
[4] Patent: WO2006/83003, 2006, A1. Location in patent: Page/Page column 36-37
[5] Journal of Medicinal Chemistry, 2017, vol. 60, # 11, p. 4680 - 4692
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW0218944287303 | N-Boc-4-甲基-4-哌啶甲酸乙酯 1-tert-Butyl 4-ethyl 4-methylpiperidine-1,4-dicarboxylate | 189442-87-3 | 5g | 211元 |
| 2025/12/22 | XW0218944287302 | N-Boc-4-甲基-4-哌啶甲酸乙酯 1-tert-Butyl 4-ethyl 4-methylpiperidine-1,4-dicarboxylate | 189442-87-3 | 1g | 50元 |
| 2025/12/22 | XW0218944287301 | N-Boc-4-甲基-4-哌啶甲酸乙酯 1-tert-Butyl 4-ethyl 4-methylpiperidine-1,4-dicarboxylate | 189442-87-3 | 250mg | 28元 |

