190791-29-8
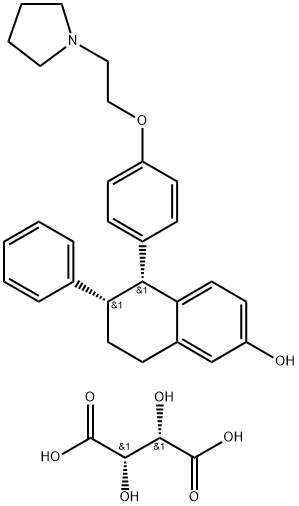 190791-29-8 结构式
190791-29-8 结构式
基本信息
酒石酸拉锁昔酚以及各个中间体杂质
Cb 336156eb
Unii-85X09V2gso
LASOFOXIFENE HCL
CP-336,156 tartrate
Lasofoxifene tartrate
(-)-cis-6-Phenyl-5-[4-(2-pyrrolidin-1-ylethoxy)phenyl]-5,6,7,8-tetrahydronaphthalen-2-ol tartrate
(5R,6S)-5,6,7,8-Tetrahydro-6-phenyl-5-[4-[2-(1-pyrrolidinyl)ethoxy]phenyl]-2-naphthalenol tartrate
(5R,6S)-5,6,7,8-Tetrahydro-6-phenyl-5-[4-[2-(1-pyrrolidinyl)ethoxy]phenyl]-2-naphthalenol (2S,3S)-2,3-dihydroxybutanedioate
(5R,6S)-5,6,7,8-Tetrahydro-6-phenyl-5-[4-[2-(1-pyrrolidinyl)ethoxy]phenyl]-2-naphthalenol (2S,3S)-2,3-dihydroxybutanedioate (1:1)
物理化学性质
制备方法

147-71-7

180916-16-9

190791-29-8
以D-酒石酸和(5R,6S)-6-苯基-5-(4-(2-(吡咯烷-1-基)乙氧基)苯基)-5,6,7,8-四氢萘-2-醇为原料,合成(5R,6S)-6-苯基-5-(4-(2-(吡咯烷-1-基)乙氧基)苯基)-5,6,7,8-四氢萘-2-酚 (2S,3S)-2,3-二羟基琥珀酸盐的一般步骤如下:将步骤d纯化的拉索昔芬(2g)溶解于20mL 95%乙醇中,与溶解于7.8mL 95%乙醇的0.78g D-酒石酸混合。将混合溶液缓慢加热至轻微回流5分钟,随后冷却至室温。得到约1.4g拉索昔芬D-酒石酸盐沉淀(产率50%)。将拉索昔芬D-酒石酸盐溶解于DMSO-d6(50mg/mL)中,用于NMR分析。1H NMR(600MHz,DMSO-d6)δ1.71-1.85(m,5H),2.08(s,1H),2.90-2.99(m,6H),3.17(br,2H),3.29(m,1H),4.02-4.06(m,4H),4.17(d,J=3.6Hz,1H),4.43(br,2H),6.6(m,5H),6.81(d,J=6.4Hz,2H),7.11(s,1H),7.13(s,2H)。13C NMR(150MHz,DMSO-d6)δ174.26,155.74,155.36,144.12,137.08,135.37,131.09,130.97,130.04,127.83,127.61,125.87,114.35,113.58,112.91,71.90,64.07,53.59,53.10,49.40,44.41,29.24,22.56,22.41。
参考文献:
[1] Patent: WO2018/129645, 2018, A1. Location in patent: Paragraph 0140; 0146-0147
