203662-51-5
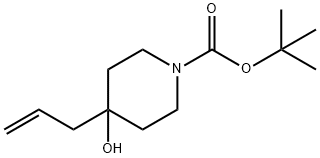 203662-51-5 结构式
203662-51-5 结构式
基本信息
1-BOC-4-烯丙基-4-羟基哌啶
4-羟基-4-(2-丙烯基)哌啶-1-羧酸叔丁酯
4-Allyl-1-Boc-4-hydroxypiperidine
benzyl 4-allyl-4-hydroxypiperidine-1-carboxylate
tert-butyl 4-allyl-4-hydroxypiperidine-1-carboxylate
tert-butyl 4-hydroxy-4-(prop-2-en-1-yl)piperidine-1-carboxyl...
tert-butyl 4-hydroxy-4-(prop-2-en-1-yl)piperidine-1-carboxylate
4-Hydroxy-4-(2-propenyl)piperidine-1-carboxylic acid tert-butyl ester
1-Piperidinecarboxylic acid, 4-hydroxy-4-(2-propenyl)-, 1,1-diMethylethyl ester
1-Piperidinecarboxylic acid, 4-hydroxy-4-(2-propen-1-yl)-, 1,1-diMethylethyl ester
物理化学性质
安全数据
toxic compounds or compounds which causing chronic effects
制备方法

79099-07-3

106-95-6

203662-51-5
a. 在氮气保护下,将N-叔丁氧羰基-4-哌啶酮(50 g, 0.25 mol)和锌粉(49.2 g, 0.75 mol)悬浮于四氢呋喃(500 mL)和氯化铵水溶液(2/3, 500 mL)的混合溶液中。控制反应温度在-10至10℃之间,缓慢滴加3-溴-1-丙烯(109.3 g, 0.90 mol)。滴加完毕后,缓慢升温至15-40℃,继续搅拌反应2-5小时。通过薄层色谱(TLC,展开剂比例为石油醚/乙酸乙酯=2/1,体积比)监测反应进度。反应完成后,用乙酸乙酯(100 mL×3)萃取反应混合物。合并有机相,用无水硫酸钠干燥,过滤后减压浓缩,得到粗产物。粗产物通过硅胶柱色谱纯化(洗脱剂比例为石油醚/乙酸乙酯=30/1至10/1,体积比),得到无色油状目标化合物4-烯丙基1-Boc-4-羟基哌啶(60 g),收率为99.9%。
参考文献:
[1] Patent: CN105440046, 2016, A. Location in patent: Paragraph 0006
[2] Patent: WO2010/58318, 2010, A1. Location in patent: Page/Page column 25-26
[3] Patent: WO2014/139144, 2014, A1. Location in patent: Page/Page column 165
[4] Patent: US2014/288081, 2014, A1. Location in patent: Paragraph 0667; 0668; 0669
[5] Patent: WO2014/139325, 2014, A1. Location in patent: Page/Page column 178; 179
