21038-22-2
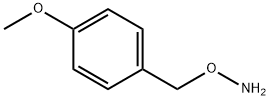 21038-22-2 结构式
21038-22-2 结构式
基本信息
HA966标准品002
HA966标准品1-2
4-甲氧基苄氧胺 10G
HA966-001-2
O-(4-Methoxybenzyl)
O-(4-Methoxybenzyl)hydroxylaMine
O-[(4-methoxyphenyl)methyl]Hydroxylamine
Hydroxylamine, O-[(4-methoxyphenyl)methyl]-
O-[(4-methoxyphenyl)methyl]Hydroxylamine ISO 9001:2015 REACH
物理化学性质
制备方法

2014-61-1

21038-22-2
以N-(4-甲氧基苄氧基)邻苯二甲酰亚胺为原料合成4-甲氧基苄氧胺的一般步骤:向含有N-(4-甲氧基苄氧基)邻苯二甲酰亚胺(0.7 mmol,236 mg)的乙醇(7 mL)溶液中加入水合肼(0.77 mmol,0.04 mL)。将反应混合物在80℃下搅拌1小时。反应完成后(通过薄层色谱法监测),将所得混合物在真空下浓缩。将残余物溶解于乙醚中,过滤并用乙醚洗涤两次。将滤液在真空下浓缩,得到4-甲氧基苄氧胺,为无色油状物(138 mg,收率95%)。1H NMR(400 MHz,CDCl3)δ 7.31(d,J = 8.0 Hz,2H),6.92(d,J = 7.9 Hz,2H),5.36(s,2H),4.63(s,2H),4.16(t,J = 4.7 Hz,2H),3.60(t,J = 4.8 Hz,2H)。13C NMR(100 MHz,CDCl3)δ 158.1,130.2,130.1,114.6,77.5,67.0,50.1。
参考文献:
[1] Bioorganic and Medicinal Chemistry Letters, 2016, vol. 26, # 10, p. 2434 - 2437
[2] Journal of Polymer Science, Part A: Polymer Chemistry, 2010, vol. 48, # 16, p. 3553 - 3563
[3] Molecules, 2013, vol. 18, # 4, p. 3872 - 3893
[4] Bioorganic and Medicinal Chemistry Letters, 2015, vol. 25, # 3, p. 607 - 610
[5] Bioorganic and Medicinal Chemistry Letters, 2003, vol. 13, # 19, p. 3155 - 3159