211308-82-6
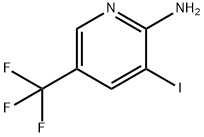 211308-82-6 结构式
211308-82-6 结构式
基本信息
3-碘-5-(三氟甲基)吡啶-2-胺
2-氨基-3-碘-5-(三氟甲基)吡啶
3-iodo-5-(trifluoromethyl)pyridine-2-amine
-iodo-5-(trifluoromethyl)-2-pyridinylamine
3-Iodo-5-trifluoroMethyl-pyridin-2-ylaMine
3-iodo-5-(trifluoromethyl)-2-pyridinylamine
2-Pyridinamine, 3-iodo-5-(trifluoromethyl)-
2-Amino-3-iodo-5-(trifluoromethyl)-pyridine
3-Iodo-5-(trifluoromethyl)-2-pyridinylamine(en)
1H-Imidazole-4-carboxylicacid,1-(7-bromophenyl)-
3-IODO-5-TRIFLUOROMETHYL-PYRIDIN-2-YLAMINE (NOT RESTRICTED/P - ROHIBITED) (LAB RESEARCH CHEMICAL)
物理化学性质
制备方法

74784-70-6

211308-82-6
在氩气保护及室温条件下,向搅拌中的5-(三氟甲基)吡啶-2-胺(100 g,617 mmol)的乙酸(1 L)溶液中依次加入浓硫酸(5 mL)、高碘酸(26 g,123 mmol)和碘(62 g,246 mmol)。将反应混合物加热至80℃并持续搅拌16小时。通过薄层色谱(TLC)监测反应进度,确认原料消耗完全后,将反应混合物冷却至0℃,并用氢氧化钠溶液(200 mL)碱化,析出固体产物。过滤收集固体,经真空浓缩后,得到目标化合物3-碘-5-(三氟甲基)吡啶-2-胺(120 g,产率67%),为灰白色固体。产物经1H NMR(DMSO-d6,500 MHz)表征:δ 8.27(s,1H),8.16(s,1H),6.87(brs,2H);TLC条件:10%乙酸乙酯/己烷(Rf 0.4)。
参考文献:
[1] Patent: WO2008/79346, 2008, A1. Location in patent: Page/Page column 58
[2] Patent: WO2017/31325, 2017, A1. Location in patent: Paragraph 0522
[3] Patent: WO2017/133667, 2017, A1. Location in patent: Page/Page column 116; 117
[4] Journal of Medicinal Chemistry, 2013, vol. 56, # 3, p. 1160 - 1170
[5] Patent: US2005/277681, 2005, A1. Location in patent: Page/Page column 26
