2166-31-6
 2166-31-6 结构式
2166-31-6 结构式
基本信息
6-苯基-3-哒嗪酮
6-苯基-3(2H)-哒嗪酮
6-苯基哒嗪-3(2H)-酮
6-苯基哒嗪-3(2H)-酮 5G
6-苯基-3(2H)-哒嗪酮
6-苯基-3-哒嗪酮(CAS号:2166-31-6)
3-Phenyl-6-pyridazinone
6-Phenyl-2H-pyridazin-3-one
6-phenylpyridazin-3(2H)-one
3-phenyl-1H-pyridazin-6-one
6-PHENYL-3(2H)-PYRIDAZINONE
3-Hydroxy-6-phenylpyridazine
3(2H)-Pyridazinone, 6-phenyl-
6-Phenyl-3(2H)-pyridazinone>
6-Phenyl-3(2H)-pyridazinone
物理化学性质
安全数据
Skin Irrit. 2
STOT SE 3
制备方法
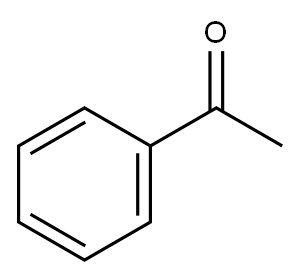
98-86-2

298-12-4

2166-31-6
将19.6 g (162.95 mmol)苯乙酮和5 g (54.32 mmol)草酰乙酸一水合物在100℃下搅拌反应2小时。反应完成后,将反应液冷却至40℃,随后加入20 mL水和4 mL氨水。用50 mL二氯甲烷对混合物进行两次萃取。向得到的水相中加入2.64 mL (53.32 mmol)一水合肼,将混合物在100℃下继续搅拌反应2小时。反应结束后,将反应液冷却至室温。通过抽滤收集沉淀的晶体,用水洗涤后,在50℃的真空干燥箱中干燥过夜。最终得到4.3 g (24.97 mmol,产率15%) 6-苯基-3-哒嗪酮,为无色晶体。LC-MS (方法7): Rt = 1.39 min; m/z = 173 (M + H)+。1H-NMR (400 MHz, DMSO-d6, δ/ppm): 13.2 (s, 1H), 8.04 (d, 1H), 7.86 (d, 2H), 7.53-7.41 (m, 3H), 7.00 (d, 1H)。
参考文献:
[1] Patent: US2011/34450, 2011, A1. Location in patent: Page/Page column 103
[2] Patent: US2012/28971, 2012, A1. Location in patent: Page/Page column 24-25
[3] Synthesis, 1993, # 3, p. 334 - 342
[4] Medicinal Chemistry Research, 2013, vol. 22, # 6, p. 2553 - 2560
[5] Letters in Drug Design and Discovery, 2013, vol. 10, # 6, p. 507 - 514
