22246-12-4
 22246-12-4 结构式
22246-12-4 结构式
基本信息
6-甲氧基-3,4-二氢异喹啉-1(2H)-酮
6-甲氧基-3,4-二氢-异喹啉-1-酮 1G
6-(甲氧基)-3,4-二氢-1(2H)-异喹啉酮
6-METHOXY-3,4-DIHYDROISOQUINOLIN-1(2H)-ONE
105298
in-1(2H)-one
6-Methoxy-3,4-dihydroisoquinoL
6-METHOXY-3,4-1(1H)-ISOQUINOLINONE
6-methoxy-3,4-dihydroisoquinolin-1-ol
3,4-Dihydro-6-methoxy-1(2H)-isoquinolinone
6-METHOXY-3,4-DIHYDRO-1(2H)-ISOQUINOLINONE
6-METHOXY-3,4-DIHYDRO-2H-ISOQUINOLIN-1-ONE
物理化学性质
制备方法
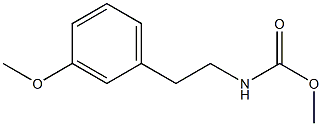
110192-21-7

22246-12-4
以化合物(CAS:110192-21-7)为原料合成6-(甲氧基)-3,4-二氢-1(2H)-异喹啉酮的一般步骤:在120℃下,向预热的多磷酸(184 mL,183.99 mmol)中缓慢加入原料化合物(35.0 g,167.26 mmol),保持温度为120℃。将反应混合物在室温下搅拌6小时,随后用冰水淬灭反应,用K2CO3碱化至pH中性,并用二氯甲烷(DCM)萃取。合并有机层,用无水Na2SO4干燥,过滤后浓缩溶剂。通过硅胶柱色谱法纯化粗产物,使用乙酸乙酯(EtOAc)/己烷(1:1)作为洗脱剂,得到目标产物6-(甲氧基)-3,4-二氢-1(2H)-异喹啉酮(38.5 g,218 mmol,收率76%),为白色固体。1H NMR(500 MHz,CDCl3)δ 8.01(d,J = 8.7 Hz,1H),6.90(d,J = 8.7 Hz,1H),6.86(dd,J = 8.7, 2.4 Hz,1H),6.71(d,J = 2.4 Hz,1H),3.85(s,3H),3.56(td,J = 6.6, 2.8 Hz,2H),2.96(t,J = 6.6 Hz,2H);EI/MS m/z 177.1 [M+].
参考文献:
[1] Bioorganic and Medicinal Chemistry, 2016, vol. 24, # 2, p. 207 - 219
[2] Patent: US2017/145007, 2017, A1. Location in patent: Paragraph 0322-0325
[3] Bioorganic and Medicinal Chemistry, 2016, vol. 24, # 4, p. 789 - 801
[4] Patent: WO2012/97682, 2012, A1. Location in patent: Page/Page column 134
[5] Patent: WO2012/97684, 2012, A1. Location in patent: Page/Page column 108
