23579-79-5
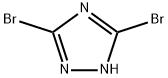
 23579-79-5 结构式
23579-79-5 结构式
基本信息
1-甲基-3,5-二溴-1,2,4-三唑
3,5-二溴-1-甲基-1,2,4-三氮唑
1-甲基-3,5-二溴-1,2,4-三氮唑
3,5-二溴-1-甲基-1,2,4-1H-三氮唑
Methyl-3,5-dibroMo-1,2,4-triazole
1-Methyl-3,5-dibroMo-1,2,4-triazole
3,5-dibromo-1-methyl-1,2,4-triazole
1-Methyl-3,5-dibroMo-1H-1,2,4-triazole
3,5-DIBROMO-1-METHYL-1H-1,2,4-TRIAZOLE
1H-1,2,4-Triazole, 3,5-dibromo-1-methyl-
物理化学性质
制备方法
7411-23-6
74-88-4

23579-79-5
在250 mL圆底烧瓶中,将3,5-二溴-1H-1,2,4-三唑(8.00 g,35.3 mmol)溶解于N,N-二甲基甲酰胺(DMF,80 mL)中,并将溶液冷却至0-5°C(冰浴)。分批加入氢化钠(60%矿物油分散体,1.69 g,42.3 mmol),将反应混合物在0-5°C下搅拌45分钟,随后在室温下搅拌15分钟。在0-5°C(冰浴)条件下,缓慢滴加碘甲烷(10.0 g,4.41 mL,70.5 mmol)。滴加完毕后,将反应混合物在室温下搅拌18小时。反应完成后,将混合物在减压下浓缩,残余物用乙酸乙酯(200 mL)和水(200 mL)稀释,分离水相并用乙酸乙酯(2×200 mL)萃取。合并有机层,用无水硫酸钠干燥,减压浓缩,得到3,5-二溴-1-甲基-1,2,4-三唑,为浅黄色固体(8.15 g,收率96%)。产物经1H NMR(CDCl3, 300 MHz)表征:δ 3.89(s, 3H)。质谱(ES+)m/z: 240.0, 242.0, 244.0 [M + H,2 Br同位素]。
参考文献:
[1] Patent: WO2018/87018, 2018, A1. Location in patent: Page/Page column 55
[2] Bioorganic and Medicinal Chemistry Letters, 2015, vol. 25, # 13, p. 2679 - 2685
[3] Patent: WO2014/195321, 2014, A1. Location in patent: Page/Page column 16; 17
[4] Patent: WO2014/195322, 2014, A1. Location in patent: Page/Page column 16
[5] Bioorganic and Medicinal Chemistry Letters, 2014, vol. 24, # 24, p. 5805 - 5813
