24623-65-2
 24623-65-2 结构式
24623-65-2 结构式
基本信息
3-叔丁基-2-羟基甲醛
3-叔丁基水杨醛 100G
2-羟基-3-叔丁基苯甲醛
3-叔丁基-2-羟基苯甲醛
3-tert-Butylsalicylaldehyde >
3-t-Butyl-2-hydroxybenzaldehyde
3-tert-butyl salicylic aldehyde
3-tert-butyl-2-hydroxybenzaldehy
2-Hydroxy-3-tert-butylbenzaldehyde
3-TERT-BUTYL-2-HYDROXYBENZALDEHYDE
3-tert-Butyl-2-hydroxybenzaldehyde 96%
001ERT-BUTYL-2-HYDROXYBENZALDEHYDE, 96%
Benzaldehyde, 3-(1,1-diMethylethyl)-2-hydroxy-
物理化学性质
安全数据
Skin Irrit. 2
STOT SE 3
制备方法
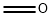
50-00-0

88-18-6

24623-65-2
在室温下,向搅拌的2-叔丁基苯酚(4.55 g,30 mmol)、氯化镁(5.71 g,60 mmol)和多聚甲醛(2.08 g,66 mmol)的THF(120 mL)悬浮液中逐滴加入三乙胺(8.35 mL,60 mmol)。将反应混合物加热至回流,持续搅拌3小时,反应完成后得到橙色悬浮液。粗产物用EtOAc(3×50 mL)萃取。若形成永久性乳液,可加入少量稀释的HCl进行破乳。合并有机层,用无水MgSO4干燥,过滤后减压浓缩,得到浅黄色油状产物,无需进一步纯化。该产物在储存过程中可能逐渐变为深绿色。产率:90%。产物为淡黄色油状物。1H-NMR (CDCl3, 300 MHz) δH: 1.44 (9H, s, 3×CH3), 6.97 (1H, t, J = 7.5 Hz, HAr), 7.41 (1H, dd, J = 1.5 Hz, J = 7.5 Hz, HAr), 7.54 (1H, dd, J = 1.2 Hz, J = 7.5 Hz, HAr), 9.88 (1H, s, CHO), 11.82 (1H, s, OH)。
参考文献:
[1] Journal of Medicinal Chemistry, 2007, vol. 50, # 7, p. 1658 - 1667
[2] Advanced Synthesis and Catalysis, 2017, vol. 359, # 22, p. 3990 - 4001
[3] Patent: WO2008/132474, 2008, A1. Location in patent: Page/Page column 31
[4] Advanced Synthesis and Catalysis, 2009, vol. 351, # 9, p. 1325 - 1332
[5] Patent: WO2009/109765, 2009, A1. Location in patent: Page/Page column 27; 28
