261165-05-3
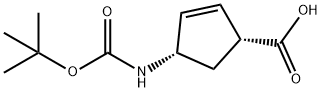
 261165-05-3 结构式
261165-05-3 结构式
基本信息
(1S,3R)-3-((叔丁氧羰基)氨基)环戊酸
(-)-(1R,3S)-BOC-3-氨基环戊烷甲酸
(1S,3R)-3((叔丁氧基羰基)氨基)环戊烷甲酸
(1R,3S)-N-BOC-1-氨基环戊烷-3-甲酸
(+)-(1S,3R)-N-BOC-3-氨基环戊烷甲酸
(+)-(1S,3R)-N-BOC-3-氨基环戊烷羧酸
(1S,3R)-(+)-3-(BOC-氨基)环戊烷羧酸
(1R,3S)-N-叔丁氧羰基-1-氨基环戊烷-3-羧酸
(1R,3S)-N-叔丁氧羰基-1-氨基环戊烷-3-甲酸
(+)-(1S,3R)-N-BOC-BETA-HOMOCYCLOLEUCINE
BOC-(1S,3R)-3-Aminocyclopentanecarboxylic acid
(1S,3R)-Boc-3-aminocyclopentane-1 carboxylic acid
(1R,3S)-N-BOC-1-AMINOCYCLOPENTANE-3-CARBOXYLIC ACID
(+)-(1S,3R)-N-BOC-3-AMINOCYCLOPENTANECARBOXYLIC ACID
(1S,3R)-(+)-3-(BOC-AMINO)CYCLOPENTANECARBOXYLIC ACID
(+)-(1R,3S)-N-BOC-1-AMINOCYCLOPENTANE-3-CARBOXYLIC ACID
(-)-(1R,3S)-N-BOC-1-AMINOCYCLOPENTANE-3-CARBOXYLIC ACID
(1R,3S)-N-boc-1-Aminocyclopentane-3-carboxylicacid(e.e.)
物理化学性质
安全数据
制备方法
151907-80-1

261165-05-3
以(1R,4S)-4-((叔丁氧羰基)氨基)环戊-2-烯甲酸为起始原料,合成(1S,3R)-3-((叔丁氧基羰基)氨基)环戊烷甲酸的一般步骤如下:将步骤A中制得的(1R,4S)-4-((叔丁氧羰基)氨基)环戊-2-烯甲酸(230g,1.0mol)与10%Pd/C催化剂(5.0g)置于500mL甲醇中,在Parr振荡器上于50psi氢气压力下氢化1小时。反应完成后,通过过滤移除催化剂,随后蒸发滤液以去除溶剂。将所得残余物溶解于二氯甲烷中,加入无水硫酸钠进行干燥。再次过滤后,蒸发滤液,并在真空条件下干燥,得到(1S,3R)-3-((叔丁氧基羰基)氨基)环戊烷甲酸,为浅黄色固体(230g,收率99%)。经LC-MS分析,C11H19NO4的[M+H+]计算值为230,实测值为230,与预期相符。
参考文献:
[1] Patent: WO2004/76411, 2004, A2. Location in patent: Page 28
[2] Patent: WO2003/93231, 2003, A2. Location in patent: Page/Page column 56
[3] Patent: US2007/155731, 2007, A1. Location in patent: Page/Page column 27; 36
[4] Patent: WO2005/110409, 2005, A2. Location in patent: Page/Page column 30
[5] Patent: WO2005/75426, 2005, A1. Location in patent: Page/Page column 28
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/09/19 | 36183 | (1S,3R)-(+)-3-(Boc-氨基)环戊烷羧酸 (1R,3S)-N-BOC-1-Aminocyclopentane-3-carboxylic acid, 95%, 98% ee, Thermo Scientific Chemicals | 261165-05-3 | 1g | 7892元 |
| 2025/05/22 | 36183 | (1S,3R)-(+)-3-(Boc-氨基)环戊烷羧酸 (1R,3S)-N-BOC-1-Aminocyclopentane-3-carboxylic acid, 95%, 98% ee, Thermo Scientific Chemicals | 261165-05-3 | 250mg | 2662元 |
| 2025/05/22 | XW2611655302 | (1S,3R)-3((叔丁氧基羰基)氨基)环戊烷甲酸 | 261165-05-3 | 1G | 158元 |
