274693-53-7
 274693-53-7 结构式
274693-53-7 结构式
基本信息
替卡格雷中间体C2
替格瑞洛杂质138
替格瑞洛起始物料G杂质8
N-[(3AS,4R,6S,6AR)-四氢-6-羟基-2,2-二甲基-4H-环戊烯并-1,3-二氧戊
N-[(3AS,4R,6S,6AR)-四氢-6-羟基-2,2-二甲基-4H-环戊烯并-1,3-二氧戊环-4-基]氨基甲酸苄酯
CarbaMic acid,N-[(3aS,4R,6S,6aR)-tetrahydro-6-hydroxy-2,2-
Benzyl [(3aS,4R,6S,6aR)-6-hydroxy-2,2-dimethyltetra-hydro-3aH-cyclopenta[d][1,3]dioxol-4-yl]ca
benzyl N-[(3aS,4R,6S,6aR)-6-hydroxy-2,2-dimethyl-hexahydrocyclopenta[d][1,3]dioxol-4-yl]carbamate
benzyl((3aS,4R,6S,6aR)-6-hydroxy-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)carbamate
Benzyl((3aS, 4R, 6S, 6aR)-6-hydroxy-2,2-diMethyltetrahydro-3aH-cyclopenta[1,3]-dioxol-4yl)carbaMate
((3AS 4R 6S 6AR)6-HYDROXY-2,2-DIMETHYL-TETRAHYDROCYCLOPENTA(1,3)DIOXOL 4,YL)CARBAMIC ACID BENZYL ESTER
N-[(3aS,4R,6S,6aR)-tetrahydro-6-hydroxy-2,2-diMethyl-4H-cyclopenta-1,3-dioxol-4-yl]-,phenylMethyl ester
N-[(3aS,4R,6S,6aR)-Tetrahydro-6-hydroxy-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-yl]carbamicacid phenylmethyl ester
Phenylmethyl ester N-[(3aS,4R,6S,6aR)-tetrahydro-6-hydroxy-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-yl]-Carbamic acid
物理化学性质
制备方法
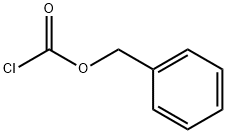
501-53-1

155899-66-4
![N-[(3AS,4R,6S,6AR)-四氢-6-羟基-2,2-二甲基-4H-环戊烯并-1,3-二氧戊环-4-基]氨基甲酸苄酯](/CAS/GIF/274693-53-7.gif)
274693-53-7
步骤7: 在0℃条件下,向(3aR,4S,6R,6aS)-6-氨基-2,2-二甲基四氢-3aH-环戊二烯并[d][1,3]二氧杂环戊烯-4-醇(17.0g,98.15mmol,1.00当量)和碳酸钠(20.8g,196.24mmol,2.00当量)的四氢呋喃:水(5:1,600mL)混合溶液中,缓慢加入氯甲酸苄酯(18.4g,107.86mmol,1.05当量)。反应完成后,用乙酸乙酯(3×200mL)进行萃取,合并有机相,经标准后处理得到标题化合物N-[(3aS,4R,6S,6aR)-四氢-6-羟基-2,2-二甲基-4H-环戊烯并-1,3-二氧戊环-4-基]氨基甲酸苄酯,为白色固体(20.5g,产率68%)。产物经1H NMR(300MHz,CDCl3)表征,δ:7.30-7.39(m,5H),5.20(s,2H),4.60(d,J=5.4Hz,1H),4.50(d,J=5.4Hz,1H),4.27(s,1H),4.19(d,J=5.7Hz,1H),2.26(m,1H),1.71(d,J=14.4Hz,1H),1.47(s,3H),1.28(s,3H)。
参考文献:
[1] Bioorganic and Medicinal Chemistry Letters, 2007, vol. 17, # 21, p. 6013 - 6018
[2] Patent: WO2011/17108, 2011, A2. Location in patent: Page/Page column 38
[3] Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 11, p. 3598 - 3602
[4] Patent: WO2012/138981, 2012, A2. Location in patent: Page/Page column 38
[5] Patent: CN106279095, 2017, A. Location in patent: Paragraph 0023; 0046
常见问题列表
N-[(3AS,4R,6S,6AR)-四氢-6-羟基-2,2-二甲基-4H-环戊烯并-1,3-二氧戊环-4-基]氨基甲酸苄酯是一种有用的手性构建基。通过氧化/立体选择性还原合成了替格瑞洛的全顺式碳环前体。
N-[(3AS,4R,6S,6AR)-四氢-6-羟基-2,2-二甲基-4H-环戊烯并-1,3-二氧戊环-4-基]氨基甲酸苄酯为羧酸酯类衍生物,可用作医药中间体。