28027-17-0
 28027-17-0 结构式
28027-17-0 结构式
基本信息
4-羟基-7-甲氧基喹啉-3-羧基
4-羟基-7-甲氧基喹啉-3-羧酸
4-羟基-7-甲氧基-3-喹啉羧酸
4-羟基-7-甲氧基喹啉-3-羧酸基
4-羟基-7-甲氧基喹啉-3-羧基 酸
7-甲氧基-4-氧代-1,4-二氢喹啉-3-羧酸
AURORA 17949
ine-3-carboxyL
BUTTPARK 20\09-64
4-Hydroxy-7-methoxyquinoL
7-methoxy-4-oxo-1H-quinoline-3-carboxylicaci
7-Methoxy-4-hydroxyquinoline-3-carboxylic acid
4-Hydroxy-7-methoxy-3-quinolinecarboxylic acid
4-HYDROXY-7-METHOXYQUINOLINE-3-CARBOXYLIC ACID
7-methoxy-4-oxo-1H-quinoline-3-carboxylic acid
物理化学性质
制备方法
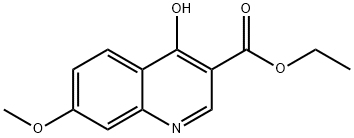
63463-15-0

28027-17-0
以4-羟基-7-甲氧基喹啉-3-甲酸乙酯为原料合成4-羟基-7-甲氧基喹啉-3-甲酸的一般步骤:将4-羟基-7-甲氧基喹啉-3-甲酸乙酯(4.40 g,17.80 mmol)溶于2 M NaOH溶液中,于110℃下回流反应1小时进行水解。反应完成后,将混合物冷却至室温,用2 M HCl溶液酸化至pH 3-4。酸化后,析出的白色沉淀经过滤收集,用水洗涤并干燥,得到目标产物4-羟基-7-甲氧基喹啉-3-甲酸(2.50 g,产率63%),熔点为272℃(文献值:Lauer等,1946,257-260℃)。核磁共振氢谱(400 MHz,DMSO-d6)δ:3.90(3H,s,OCH3),7.17(1H,dd,J = 2.4, 9.0 Hz,H-6),7.29(1H,d,J = 2.3 Hz,H-8),8.16(1H,d,J = 9.0 Hz,H-5),8.72(1H,s,H-2),13.68(1H,s,Ar-OH),15.50(1H,bs,-COOH)。核磁共振碳谱(100 MHz,DMSO-d6)δ:56.3(Ar-OCH3),101.1(C-3),107.8(C-8),116.6(C-4a),118.9(C-6),127.3(C-5),142.0(C-2),145.0(C-8a),163.8(C-4),166.9(C-7),178.1(COOH)。
参考文献:
[1] Bioorganic and Medicinal Chemistry, 2013, vol. 21, # 13, p. 3738 - 3748
[2] Journal of the American Chemical Society, 1946, vol. 68, p. 1268
[3] Journal of Medicinal Chemistry, 1998, vol. 41, # 25, p. 4918 - 4926
[4] Chemical and Pharmaceutical Bulletin, 2007, vol. 55, # 5, p. 821 - 824
[5] Patent: US6110929, 2000, A
