287944-10-9
 287944-10-9 结构式
287944-10-9 结构式
基本信息
环戊烯硼酸片呐醇酯
1-环戊烯硼酸频哪醇酯
1-环戊烯硼酸频呐醇酯
环戊烯-1-硼酸频呐醇酯
环戊烯-1-基硼酸频哪醇酯
1-环戊烯硼酸频哪醇酯 1G
2-(1-环戊烯基)-4,4,5,5-四甲基-1,3,2-二氧杂环戊硼烷
2-(环戊-1-烯-1-基)-4,4,5,5-四甲基-1,3,2-二氧杂环戊硼烷
2-CYCLOPENTENYL-4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLANE1-环戊烯硼酸频哪醇酯
1-Cyclopentenylboronic acid pinacol este
Cyclopenten-1-ylboronic acid picol ester
1-Cyclopentenyl boronic acid pinacol ester
Cyclopentene-1-boronic acid, pinacol ester
Cyclopenten-1-ylboronic acid, pinacol ester
1-Cyclopentenyl-1-yl-boronic acid pinacol ester
2-CYCLOPENTENYL-4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLANE
1-Cyclopentenyl-4,4,5,5-TetraMethyl-1,3,2-Dioxaborolane
2-(1-Cyclopentenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
物理化学性质
制备方法
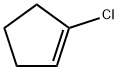
930-29-0
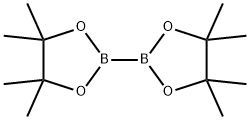
73183-34-3

287944-10-9
实施例XIV: 2-环戊-1-烯基-4,4,5,5-四甲基-[1,3,2]二氧杂硼杂环戊烷的合成 在氩气保护下,将4.31 g 1-氯-1-环戊烯和12.5 g双(频哪醇)二硼溶于160 mL 1,4-二恶烷中,加入8 g乙酸钾,氩气吹扫5分钟。随后加入370 mg三(二亚苄基乙酰基)二钯(0) ([Pd2(dba)3])和780 mg 2-环己基膦基-2',4',6'-三异丙基-1,1'-联苯,将反应混合物加热至80℃反应3小时。反应完成后,冷却至室温,用二乙醚稀释,依次用水和盐水洗涤有机相,有机相用无水硫酸镁干燥。减压蒸发除去溶剂,残余物用石油醚研磨。过滤收集沉淀,母液减压蒸发得到产物。产量:13.1 g (产率99%)。质谱(EI): m/z = 194 [M]+。
参考文献:
[1] Patent: WO2011/101424, 2011, A1. Location in patent: Page/Page column 230-231
[2] Patent: US2012/46304, 2012, A1. Location in patent: Page/Page column 123
[3] Patent: WO2012/110599, 2012, A1. Location in patent: Page/Page column 230-231
[4] Patent: US2013/53404, 2013, A1. Location in patent: Paragraph 1781; 1782; 1783
[5] Journal of Organic Chemistry, 2012, vol. 77, # 7, p. 3543 - 3548
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | C3385 | 2-(1-环戊烯基)-4,4,5,5-四甲基-1,3,2-二氧杂环戊硼烷 2-(1-Cyclopentenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane | 287944-10-9 | 1g | 100元 |
| 2025/12/22 | C3385 | 2-(1-环戊烯基)-4,4,5,5-四甲基-1,3,2-二氧杂环戊硼烷 2-(1-Cyclopentenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane | 287944-10-9 | 5g | 460元 |
| 2025/12/22 | XW0228794410901 | 1-环戊烯硼酸频哪醇酯 2-(Cyclopent-1-en-1-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane | 287944-10-9 | 1g | 28元 |
