29096-64-8
 29096-64-8 结构式
29096-64-8 结构式
基本信息
1-(咪唑并[1,2-A]吡啶-3-基)乙酮
1-(Imidazo[1,2-a]pyridin-3-yl)ethanone
Ethanone, 1-iMidazo[1,2-a]pyridin-3-yl-
Ethanone, 1-imidazo[1,2-a]pyridin-3-yl- (9CI)
物理化学性质
制备方法
![咪唑并[1,2-a]吡啶](/CAS/GIF/274-76-0.gif)
274-76-0
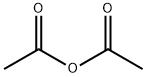
108-24-7
![1-(咪唑并[1,2-A]吡啶-3-基)乙酮](/CAS/GIF/29096-64-8.gif)
29096-64-8
在0℃及惰性气氛下,将咪唑并[1,2-a]吡啶(8.0g,67.7mmol)溶于二硫化碳(80mL)中,缓慢加入无水AlCl3(22.4g,169.4mmol)粉末。室温搅拌30分钟后,温和回流反应混合物,并在30分钟内滴加乙酸酐(6.8mL,67.7mmol)。继续在回流温度下反应5小时。减压蒸馏除去二硫化碳,残余物用冰水(200mL)淬灭。水层用二氯甲烷(2×200mL)萃取,有机相依次用饱和NaHCO3溶液(2×100mL)、水(100mL)和盐水(100mL)洗涤。有机层经无水Na2SO4干燥后减压浓缩,得到棕色固体Int-1(2.3g,22%)。1H NMR (200MHz, CDCl3): δ 9.67 (d, J = 7Hz, 1H), 8.36 (bs, 1H), 7.79 (d, J = 8.8Hz, 1H), 7.54-7.46 (m, 1H), 7.13-7.06 (m, 1H), 2.61 (s, 3H)。质谱 (m/z): 148 [M++1]。将Int-1(5g,31.2mmol)溶于DMFDMA(25mL)中,在N2保护下回流搅拌36小时。TLC监测反应完成后,冷却至室温,用乙醚(100mL)稀释并搅拌15分钟。过滤收集固体,用乙醚(2×10mL)洗涤,真空干燥得棕色固体Int-2(4.7g,70%)。1H NMR (200MHz, CDCl3): δ 9.82 (d, J = 6.8Hz, 1H), 8.22 (s, 1H), 7.81 (d, J = 12.2Hz, 1H), 7.71 (d, J = 8.8Hz, 1H), 7.37 (t, J = 7.4Hz, 1H), 6.97 (t, J = 6.2Hz, 1H), 5.71 (d, J = 12.2Hz, 1H), 3.06 (bs, 6H)。质谱 (m/z): 216.0 [M++1]。在室温及惰性气氛下,将Int-2(1.3g,6.0mmol)溶于DMF(10mL)中,加入Int-2B(3.5g,18.1mmol)和K2CO3(2.5g,18.1mmol),混合物在100℃加热16小时。冷却至室温后,倒入冰水(70mL)中搅拌15分钟。过滤收集固体,用水(10mL)洗涤,真空干燥得浅棕色固体Int-3(1.1g,55%)。1H NMR (200MHz, DMSO-d6): δ 10.11-10.05 (m, 2H), 8.64 (s, 1H), 8.52 (d, J = 5.5Hz, 1H), 7.85-7.63 (m, 5H), 7.46 (t, J = 7.0Hz, 1H), 7.32 (d, J = 5.3Hz, 1H), 7.07 (d, J = 6.2Hz, 1H), 3.75 (s, 3H)。质谱 (m/z): 345.9 [M++1]。在0℃下,将Int-3(0.7g,2mmol)溶于甲醇(35mL)和二氯甲烷(14mL)中,加入50wt%羟胺水溶液(14mL)。搅拌10分钟后,在0℃下加入NaOH溶液(0.56g,溶于3.5mL水)。反应混合物升至室温并搅拌5小时。减压蒸发挥发物,残余物用2N HCl在0℃下中和至pH≈7,搅拌10分钟。过滤收集固体,用水(2×5mL)洗涤,真空干燥。粗产物经制备型HPLC(乙腈:水:0.1%三氟乙酸)纯化,得到TFA盐形式的灰白色固体化合物5(0.22g,31%)。1H NMR (200MHz, DMSO-d6): δ 11.05 (bs, 1H), 10.2 (d, J = 6.6Hz, 1H), 10.07 (s, 1H), 8.92 (bs, 1H), 8.59 (d, J = 5.6Hz, 1H), 7.96 (d, J = 8.8Hz, 1H), 7.86-7.72 (m, 5H), 7.52 (d, J = 5.2Hz, 1H), 7.39 (t, J = 6.6Hz, 1H)。13C NMR (120MHz, DMSO-d6): δ 164.4, 159.0, 158.5, 158.2, 158.0, 156.0, 142.7, 132.1, 131.0, 130.2, 127.6, 125.7, 121.7, 118.4, 115.9, 114.9, 108.2。质谱 (m/z): 347.2 [M++1]。
参考文献:
[1] Patent: US2010/29638, 2010, A1. Location in patent: Page/Page column 65
[2] Bioorganic and Medicinal Chemistry Letters, 2004, vol. 14, # 9, p. 2245 - 2248
[3] Patent: EP1214318, 2003, B1. Location in patent: Page/Page column 33
[4] Patent: EP1790650, 2007, A1. Location in patent: Page/Page column 44
[5] Patent: CN103130792, 2016, B. Location in patent: Paragraph 0223-0225
