2955-46-6
 2955-46-6 结构式
2955-46-6 结构式
基本信息
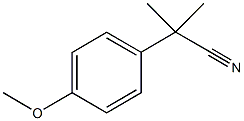
2-(4-甲氧基苯基)-2-甲基丙酸
2-(4-甲氧苯基)-2-甲基-丙酸
4-甲氧基-.ALPHA.,.ALPHA.-二甲基-苯乙酸
2-(4-Methoxyphenyl)-2-methylpropionic acid
Benzeneacetic acid,4-Methoxy-a,a-diMethyl-
Benzeneacetic acid, 4-Methoxy-α,α-diMethyl-
Benzeneacetic acid, 4-Methoxy-alpha,alpha-diMethyl-
4-methoxy-.alpha.,.alpha.-dimethyl-Benzeneacetic acid
制备方法
5351-07-5

2955-46-6
步骤24b:将2-(4-甲氧基苯基)-2-甲基丙腈(化合物0802-27,1.63g,9.30mmol)溶于乙二醇(3.40mL)中,随后加入氢氧化钾(1.29g,22.99mmol)和水(0.46mL)。将反应混合物在150℃下加热搅拌7小时。反应完成后,将混合物倒入水中,用浓盐酸调节pH至2。过滤反应混合物,用水洗涤收集的固体,并在真空下干燥,得到目标产物2-(4-甲氧基苯基)-2-甲基丙酸(化合物0803-27,0.85g,产率47%),为白色固体。产物经LCMS分析显示:[M+1]+为195。1H NMR(DMSO-d6)数据如下:δ1.44(s,6H),3.73(s,3H),6.88(d,J=9.2Hz,2H),7.25(d,J=8.4Hz,2H),12.23(s,1H)。
参考文献:
[1] European Journal of Organic Chemistry, 2007, # 21, p. 3449 - 3462
[2] Patent: WO2009/36016, 2009, A1. Location in patent: Page/Page column 77; 78
[3] Acta Chemica Scandinavica (1947-1973), 1954, vol. 8, p. 1203,1207
[4] Acta Chemica Scandinavica (1947-1973), 1955, vol. 9, p. 210,214
[5] Journal of the American Chemical Society, 1956, vol. 78, p. 4801,4805
