3177-20-6
 3177-20-6 结构式
3177-20-6 结构式
基本信息
2,4-二氯嘧啶-5-甲酸甲酯
2,4-二氯-5-嘧啶甲酸甲酯
2,4-二氯-5-嘧啶羧酸甲酯
甲基 2,4-二氯嘧啶-5-羧酸酯
Methyl 2,4-dichloropyrimidine-5-carboxylate 97%
Methyl 2,4-dichloropyrimidine-5-carboxylate, GC 97%
2,4-Dichloropyrimidine-5-carboxylicacid methyl ester
5-PyriMidinecarboxylic acid, 2,4-dichloro-, Methyl ester
Methyl2,4-Dichloropyrimidine-5-carboxylate ISO 9001:2015 REACH
物理化学性质
安全数据
Eye Irrit. 2
Skin Irrit. 2
STOT SE 3
制备方法
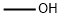
67-56-1
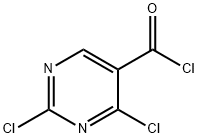
2972-52-3

3177-20-6
在0℃下,向2,4-二氯嘧啶-5-碳酰氯(500mg,2.38mmol)的二氯甲烷(30mL)溶液中缓慢加入甲醇(87.6mg,2.73mmol)和二异丙基乙胺(369mg,2.86mmol)。反应混合物在0℃下持续搅拌1小时。反应完成后,通过旋转蒸发仪除去溶剂。将得到的残余物(461mg,收率94%)溶解于异丙醇(20mL)中,随后逐滴加入反式-4-氨基环己醇(301.6mg,2.62mmol)和二异丙基乙胺(461.4mg,3.57mmol)。反应混合物在0℃下继续搅拌90分钟。接着,加入正丁胺(208.8mg,2.86mmol)和二异丙基乙胺(461.4mg,3.57mmol),并将混合物在室温下搅拌3小时。反应结束后,加入水淬灭反应。用乙酸乙酯(3×30mL)萃取反应混合物。合并有机层,用无水硫酸钠干燥,过滤后浓缩。残余物通过ISCO自动快速色谱系统纯化,得到2-(丁基氨基)-4-((反式-4-羟基环己基)氨基)嘧啶-5-羧酸甲酯(682.6mg,三步总收率89%)。1H NMR(400MHz,CDCl3)δ9.21(s,1H),8.77(s,1H),6.29(s,1H),4.81-4.64(m,1H),4.51(s,3H),4.46-4.38(m,1H),4.13-4.11(m,2H),2.89-2.81(m,2H),2.74(d,J=9.7Hz,2H),2.35-2.25(m,2H),2.23-2.00(m,6H),1.67(t,J=7.2Hz,3H);13C NMR(101MHz,CDCl3)δ167.9,162.5,161.3,160.3,95.5,69.7,51.2,48.3,41.1,33.8,31.7,30.3,20.1,13.8;MS m/z 323.20 [M+H]+。
参考文献:
[1] Patent: WO2013/177168, 2013, A1. Location in patent: Page/Page column 34; 35
[2] Patent: WO2014/85225, 2014, A1. Location in patent: Page/Page column 18; 19; 28; 29
[3] Patent: WO2015/157127, 2015, A1. Location in patent: Page/Page column 167
[4] Bioorganic and Medicinal Chemistry Letters, 2011, vol. 21, # 16, p. 4857 - 4859
[5] Bioorganic and Medicinal Chemistry Letters, 2008, vol. 18, # 16, p. 4642 - 4646
