337915-79-4
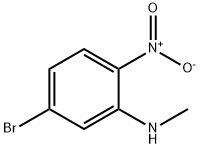
 337915-79-4 结构式
337915-79-4 结构式
基本信息
4-溴-2-甲基氨基苯胺 10G
5-溴-N1-甲基-1,2-苯二胺
5-溴-1-N-甲基苯-1,2-二胺
4-Bromo-2-methylaminoaniline
5-Bromo-N1-methyl-1,2-benzenediamine
5-Bromo-N1-methylbenzene-1,2-diamine
4-Bromo-N2-methyl-1,2-benzenediamine
4-bromo-2-N-methylbenzene-1,2-diamine
4-bromo-N2-methyl-benzene-1,2-diamine
1,2-BENZENEDIAMINE, 4-BROMO-N2-METHYL-
1,2-BENZENEDIAMINE, 4-BROMO-N2-METHYL- ISO 9001:2015 REACH
物理化学性质
制备方法
302800-13-1

337915-79-4
以5-溴-N-甲基-2-硝基苯胺(350 mg,1.51 mmol)为原料,与锌粉(495 mg,7.57 mmol)和氯化铵(810 mg,15.15 mmol)在甲醇(4 mL)和水(2 mL)的混合溶剂中,于室温下搅拌反应1小时。反应完成后,通过过滤去除不溶物,滤液用饱和碳酸氢钠溶液中和。随后,将反应混合物浓缩,并用乙酸乙酯萃取。分离有机层,依次用水和饱和食盐水洗涤,经无水硫酸镁干燥后,减压浓缩,得到4-溴-2-甲基氨基苯胺,为棕色固体(282 mg,收率93%)。产物经1H NMR(400 MHz,DMSO-d6)确认:δ 2.68(3H,d,J = 4.9 Hz),4.60(2H,s),4.87(1H,d,J = 4.9 Hz),6.32-6.58(3H,m)。质谱(ESI/APCI)显示m/z = 202.09 [M + H]+。
参考文献:
[1] Bioorganic and Medicinal Chemistry, 2016, vol. 24, # 11, p. 2486 - 2503
[2] Patent: CN108689942, 2018, A. Location in patent: Paragraph 0463; 0468-0469
[3] Patent: WO2012/174312, 2012, A2. Location in patent: Page/Page column 216-217
[4] Patent: WO2008/82487, 2008, A2. Location in patent: Page/Page column 111-112
[5] Patent: WO2004/111036, 2004, A1. Location in patent: Page 21
