364077-84-9
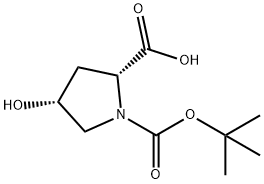
 364077-84-9 结构式
364077-84-9 结构式
基本信息
N-BOC-4-氧代-D-脯氨酸甲酯
(R)-N-BOC-4-氧代四氢吡咯-2-甲酸
(R)-1-(叔丁氧基羰基)-4-氧代吡咯烷-2-羧酸
Boc-4-Oxo-D-Proline
N-BOC-4-OXO-D-PROLINE
(2R)-N-Boc-4-oxo-proline
(2R)-N-Boc-4-oxo-D-proline
N-Boc-4-oxo-D-proline Methyl ester
(R)-4-OXO-PYRROLIDINE-1,2-DICARBOXYLIC ACID 1-TERT-BUTYL ESTER
(R)-1-(tert-Butoxycarbonyl)-4-oxopyrrolidine-2-carboxylic acid
(2R)-1-[(tert-butoxy)carbonyl]-4-oxopyrrolidine-2-carboxylic acid
(R)-4-OXO-PYRROLIDINE-1,2-DICARBOXYLIC ACID 1-TERT-BUTYL ESTER USP/EP/BP
物理化学性质
制备方法
135042-12-5

364077-84-9
步骤1:制备(R)-1-(叔丁氧基羰基)-4-氧代吡咯烷-2-羧酸(29a)。在0℃下,将(2R,4R)-1-(叔丁氧基羰基)-4-羟基吡咯烷-2-羧酸(14b)(51g,221mmol)溶于二氯甲烷(2023mL)中,加入三氯异氰尿酸(51.3g,221mmol)和TEMPO(1.723g,11.03mmol)。保持0℃搅拌30分钟,然后缓慢升温至室温并搅拌过夜。反应完成后,用水(100mL)稀释反应混合物,继续搅拌30分钟。随后,通过减压蒸馏除去二氯甲烷。将残余物用乙酸乙酯(200mL)稀释,通过硅藻土塞过滤。滤液用1M HCl(8mL)酸化,分离有机层。乙酸乙酯层依次用水(4×200mL)和饱和盐水(100mL)洗涤,经无水硫酸钠干燥后过滤,减压浓缩,得到白色固体产物(R)-1-(叔丁氧基羰基)-4-氧代吡咯烷-2-羧酸(29a)(38g,166mmol,收率75%)。产物经1H NMR(300 MHz,DMSO-d6)和质谱(ES-)确认。
参考文献:
[1] Bioorganic and Medicinal Chemistry, 2009, vol. 17, # 6, p. 2501 - 2511
[2] Patent: WO2017/59178, 2017, A1. Location in patent: Page/Page column 105
[3] Patent: WO2004/87646, 2004, A2. Location in patent: Page/Page column 63-64
[4] Patent: WO2017/117239, 2017, A1. Location in patent: Page/Page column 53; 54
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW0236407784905 | N-Boc-4-氧代-D-脯氨酸甲酯 (R)-1-(tert-Butoxycarbonyl)-4-oxopyrrolidine-2-carboxylic acid | 364077-84-9 | 5g | 574元 |
| 2025/12/22 | XW0236407784904 | N-Boc-4-氧代-D-脯氨酸甲酯 (R)-1-(tert-Butoxycarbonyl)-4-oxopyrrolidine-2-carboxylic acid | 364077-84-9 | 1g | 175元 |
| 2025/12/22 | XW0236407784902 | N-Boc-4-氧代-D-脯氨酸甲酯 (R)-1-(tert-Butoxycarbonyl)-4-oxopyrrolidine-2-carboxylic acid | 364077-84-9 | 250mg | 75元 |