38202-27-6
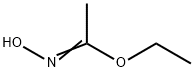
 38202-27-6 结构式
38202-27-6 结构式
基本信息
间甲苯磺酰乙酰羟胺酸乙酯
O-(2,4,6-三甲基苯磺酰基)乙酰羟肟酸乙酯[强力氨化试剂的前驱体]
O-(2-均三甲苯磺酰)乙酰羟肟酸乙酯
三甲基苯磺酰基乙酰羟肟酸乙酯
ETHYL O-MESITYLSULFONYLACETOHYDROXAMATE
O-(2-MESITYLENESULFONYL)ACETHYLDROXAMIC ACID ETHYL ESTER
O-MESITYLSULFONYLACETOHYDROXAMIC ACID ETHYL ESTER
ethyl O-methylsulphonylacetohydroxamate
O-MESITYLSULFONYLACETOHYDROXAMIC ACID ETHYL ESTER [PRECURSOR OF THE POWERFUL AMINATING REAGENT] 98+%
Ehylo-(2-mesitylenesulfonyl)acet-hydroxamate
o-(2-mesitylenesulfonyl)acethydroxamic acid ethyl ester
Ethyl O-methylsulfonylacetohydroxamate.
Ethyl O-Mesitylsulfonylacetohydroxamate [Precursor of the Powerful Aminating Reagent]
ethyl O-mesitylenesulfonylacetohydroxamate
Ethyl o-(mesitylsulfonyl)acetohydroximate
N-[[(2,4,6-Trimethylphenyl)sulfonyl]oxy]ethanimidic acid ethyl ester
O2-(Mesitylsulfonyl)acetohydroximic acid ethyl ester
Ethyl O-mesitylsulfonylaceto-hydroxamate,97%
Ethyl O-mesitylsulfonylacetohydroxamate,99%
物理化学性质
制备方法

773-64-8
10576-12-2

38202-27-6
将N-羟基乙酰亚胺酸乙酯(2.00 g,19.4 mmol)、N,N-二异丙基乙胺(3.01 g,23.3 mmol)和4-二甲基氨基吡啶(240 mg,1.96 mmol)溶解于无水二氯甲烷(30 mL)中,并将溶液冷却至0°C。在搅拌下,向冷却的溶液中缓慢加入2,4,6-三甲基苯-1-磺酰氯(4.66 g,21.3 mmol)。反应混合物逐渐升温至室温,并继续搅拌1小时。反应完成后,加入水(40 mL)淬灭反应,随后用二氯甲烷(30 mL,共3次)萃取。合并有机层,用无水硫酸钠干燥,减压浓缩得到粗产物。粗产物通过硅胶快速柱色谱法纯化,使用5%乙酸乙酯的己烷溶液作为洗脱剂,得到O-(2,4,6-三甲基苯磺酰基)乙酰羟肟酸乙酯,为白色固体(5.19 g,产率94%)。产物经1H NMR(400 MHz, CDCl3)、13C NMR(101 MHz, CDCl3)和HRMS(ESI/TOF)表征,数据与预期结构一致。
参考文献:
[1] RSC Advances, 2018, vol. 8, # 25, p. 13755 - 13763
[2] Synthetic Communications, 2018, vol. 48, # 6, p. 626 - 631
[3] Patent: WO2011/112186, 2011, A1. Location in patent: Page/Page column 202
[4] Patent: CN108530315, 2018, A. Location in patent: Paragraph 0100-0102
[5] Journal of Heterocyclic Chemistry, 2007, vol. 44, # 6, p. 1383 - 1387
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | M1182 | O-(2,4,6-三甲基苯磺酰基)乙酰羟肟酸乙酯[强力氨化试剂的前驱体] Ethyl O-Mesitylsulfonylacetohydroxamate [Precursor of the Powerful Aminating Reagent] | 38202-27-6 | 1G | 170元 |
| 2025/12/22 | M1182 | O-(2,4,6-三甲基苯磺酰基)乙酰羟肟酸乙酯[强力氨化试剂的前驱体] Ethyl O-Mesitylsulfonylacetohydroxamate [Precursor of the Powerful Aminating Reagent] | 38202-27-6 | 5G | 475元 |
| 2025/12/22 | M1182 | O-(2,4,6-三甲基苯磺酰基)乙酰羟肟酸乙酯[强力氨化试剂的前驱体] Ethyl O-Mesitylsulfonylacetohydroxamate [Precursor of the Powerful Aminating Reagent] | 38202-27-6 | 25G | 1890元 |
