39945-54-5
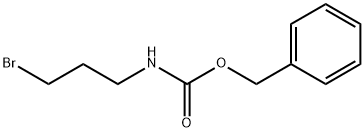 39945-54-5 结构式
39945-54-5 结构式
基本信息
3-溴丙基氨基甲酸苄酯
3-溴-N-CBZ-1-丙胺
Wln: E3mvo1r
N-BOC-3-BROMOPROPYLAMINE
3-Bromo-N-Cbz-1-propanamine
Benzyl (3-bromopropyl)carbamate
Benzyl N-(3-broMopropyl)carbaMate
TERT-BUTYL N-(3-BROMOPROPYL)CARBAMATE
(3-bromopropyl)carbamicacidbenzylester
(3-bromopropyl)-carbamicacibenzylester
N-(TERT-BUTOXYCARBONYL)-3-BROMOPROPYLAMINE
物理化学性质
制备方法

5003-71-4
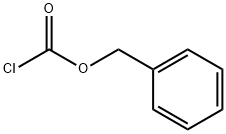
501-53-1

39945-54-5
制备例15:3-(4-(3-氯苯基)哌嗪-1-基)丙-1-胺二盐酸盐; 步骤1:3-溴丙基氨基甲酸苄酯; 在0℃下,将氯甲酸苄酯(8.6 mL,60 mmol)缓慢滴加至3-溴丙胺氢溴酸盐(6.6 g,30 mmol)溶于二氯甲烷(100 mL)和3N氢氧化钠溶液(100 mL)的搅拌混合物中。反应混合物在室温下搅拌过夜后,分离有机层,并用去离子水洗涤两次(每次30 mL)。有机层经无水硫酸镁干燥后,减压浓缩除去溶剂。残余物通过硅胶柱色谱法(洗脱剂比例:乙酸乙酯:正己烷 = 1:4)纯化,得到无色油状的目标化合物3-溴丙基氨基甲酸苄酯(8.6 g,收率98%)。1H NMR (400 MHz, CDCl3) δ 7.40-7.30 (m, 5H), 5.10 (s, 2H), 3.72 (br s, 2H), 2.25-2.18 (m, 2H), 1.98 (br, 2H), 1.81-1.78 (m, 3H). MS (ESI) m/z: [M+H]+ 272.
参考文献:
[1] Patent: WO2012/95628, 2012, A1. Location in patent: Page/Page column 20
[2] Journal of Labelled Compounds and Radiopharmaceuticals, 1996, vol. 38, # 1, p. 31 - 40
[3] Journal of Organic Chemistry, 2000, vol. 65, # 26, p. 8979 - 8987
[4] Journal of Medicinal Chemistry, 2006, vol. 49, # 17, p. 5273 - 5281
[5] Journal of Medicinal Chemistry, 2001, vol. 44, # 19, p. 3175 - 3186
