41230-93-7
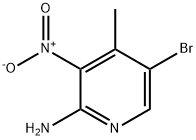
 41230-93-7 结构式
41230-93-7 结构式
基本信息
5-溴-4-甲基吡啶-2,3-二胺
2,3-二氨基-5-溴-4-甲基吡啶
5-溴-2,3-二氨基-4-甲基吡啶
5-Bromo-2,3-Diamino-4-Picoline
2,3-diamino-5-bromo-4-methylpyridine
5-bromo-4-methyl-2,3-Pyridinediamine
5-Bromo-2,3-diamino-4-methylpyridine
2,3-Diamine-4-methyl-5-bromopyridine
5-BROMO-4-METHYL-PYRIDINE-2,3-DIAMINE
2,3-Pyridinediamine, 5-bromo-4-methyl-
5-Bromo-2,3-diamino-4-methylpyridine 97%
5-BROMO-4-METHYL-PYRIDINE-2,3-DIAMINE ISO 9001:2015 REACH
物理化学性质
制备方法
100367-40-6

41230-93-7
以5-溴-4-甲基-3-硝基吡啶-2-胺(700.0 mg,3.02 mmol)为原料,加入铁粉(1680.0 mg,30.20 mmol)和浓盐酸(50.0 μL)于乙醇(2.8 mL)和水(0.7 mL)的混合溶剂中。将反应悬浮液在100℃下搅拌30分钟。反应完成后,将混合物冷却至室温,通过硅藻土过滤以去除不溶性杂质。滤液经减压蒸馏浓缩,所得残余物通过硅胶柱色谱法(洗脱剂:甲醇/二氯甲烷 = 1:40)进行纯化。收集含有目标产物的级分,经蒸发除去溶剂后,得到5-溴-4-甲基吡啶-2,3-二胺的棕色固体(550.0 mg,收率90%)。产物经LCMS ESI(+)分析:m/z 202([M + H]+),204([M + H + 2]+)。1H-NMR(300 MHz,DMSO-d6)数据:δ 7.37(s,1H),5.49(s,2H),4.74(s,2H),2.12(s,3H)。
参考文献:
[1] Patent: US2014/315888, 2014, A1. Location in patent: Paragraph 0842-0844
[2] Journal of Medicinal Chemistry, 2018, vol. 61, # 7, p. 2949 - 2961
[3] Journal of Medicinal Chemistry, 2010, vol. 53, # 22, p. 7958 - 7966
[4] Journal of the American Chemical Society, 1957, vol. 79, p. 6421,6423,6424
[5] Bioorganic and Medicinal Chemistry Letters, 1996, vol. 6, # 22, p. 2749 - 2754
