473927-64-9
中文名称
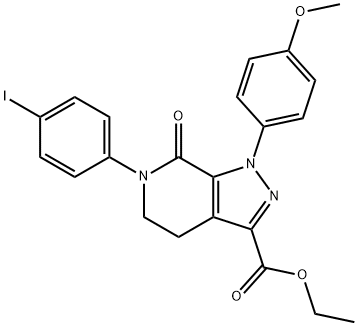
6-(4-碘苯基)-1-(4-甲氧基苯基)-7-氧代-4,5,6,7-四氢-1H-吡唑并[3,4-C]吡啶-3-羧酸乙酯
英文名称
1-(4-Methoxyphenyl)-6-(4-iodophenyl)-7-oxo-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxylic acid ethyl ester
CAS
473927-64-9
分子式
C22H20IN3O4
分子量
517.32
MOL 文件
473927-64-9.mol
 473927-64-9 结构式
473927-64-9 结构式
基本信息
中文别名
阿哌沙班杂质107(4-碘苯基)-1-(4-甲氧基苯基)-7-氧代-4,5,6,7-四氢-1H-吡
6-(4-碘苯基)-1-(4-甲氧基苯基)-7-氧代-4,5,6,7-四氢-1H-吡唑并[3,4-C]吡啶-3-羧酸
6-(4-碘苯基)-1-(4-甲氧基苯基)-7-氧代-4,5,6,7-四氢-1H-吡唑并[3,4-C]吡啶-3-羧酸乙酯
6-(4-碘苯基)-1-(4-甲氧基苯基)-7-氧代-4,5,6,7-四氢-1H-吡唑并[3,4-C]吡啶-3-羧酸乙酯,阿哌沙班中间体
6-(4-碘苯基)-1-(4-甲氧基苯基)-7-氧代-4,5,6,7-四氢-1H-吡唑并[3,4-C]吡啶-3-羧酸乙酯,阿哌沙班中间体 10G
英文别名
Apixaban IVApixaban Impurity 107
AXN-IIIQ: What is AXN-III Q: What is the CAS Number of AXN-III
Ethyl 6-(4-iodophenyl)-1-(4-methoxyphenyl)-7-oxo-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine
ethyl 6-(4-iodophenyl)-1-(4-methoxyphenyl)-7-oxo-4,5-dihydropyrazolo[3,4-c]pyridine-3-carboxylate
Ethyl 6-(4-iodophenyl)-1-(4-methoxyphenyl)-7-oxo-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-
1-(4-Methoxyphenyl)-6-(4-iodophenyl)-7-oxo-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxyli
6-(4-iodophenyl)-1-(4-Methoxyphenyl)-7-oxo-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxylate
Ethyl 6-(4-iodophenyl)-1-(4-methoxyphenyl)-7-oxo-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxylate
1-(4-Methoxyphenyl)-6-(4-iodophenyl)-7-oxo-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxylic acid ethyl este
所属类别
医药中间体:吡啶类化合物物理化学性质
沸点649.4±55.0 °C(Predicted)
密度1.60
储存条件2-8°C(protect from light)
酸度系数(pKa)-0.78±0.20(Predicted)
外观Off-white to yellow Solid
制备方法
方法1

473927-69-4
![[(4-甲氧基苯基)肼基]氯乙酸乙酯](/CAS2/GIF/27143-07-3.gif)
27143-07-3
![6-(4-碘苯基)-1-(4-甲氧基苯基)-7-氧代-4,5,6,7-四氢-1H-吡唑并[3,4-C]吡啶-3-羧酸乙酯](/CAS2/GIF/473927-64-9.gif)
473927-64-9
在惰性气氛(氩气)下进行反应。首先,将[(4-甲氧基苯基)亚肼基]氯乙酸乙酯(14.1 g, 0.055 mol)溶解于140 mL乙酸乙酯中。将溶液置于冰水浴中冷却至0-5℃。在搅拌条件下,分批加入1-(4-碘苯基)-3-吗啉-5,6-二氢吡啶-2(1H)-酮(21.1 g, 0.055 mol)。随后,在0-5℃下缓慢滴加三乙胺(11.1 g, 0.110 mol)。停止冷却,让反应混合物升温至室温。接着,加热反应混合物至回流状态,并维持回流约1小时。反应进程通过HPLC监测,确保反应在120分钟内完成。再次将反应混合物冷却至0-5℃,缓慢滴加由浓盐酸(27.5 mL, 0.275 mol)与等体积蒸馏水稀释的1:1盐酸溶液。冷却后,继续搅拌反应混合物约1小时。然后,加入55 mL水,将形成的悬浮液在冷却条件下继续搅拌2小时。通过过滤分离产物,并在50℃下真空干燥24小时,得到6-(4-碘苯基)-1-(4-甲氧基苯基)-7-氧代-4,5,6,7-四氢-1H-吡唑并[3,4-c]吡啶-3-羧酸(产物III),产量21.3 g,产率75%,HPLC纯度为95%。
参考文献:
[1] Patent: WO2014/75648, 2014, A1. Location in patent: Page/Page column 11
[2] Patent: WO2015/177801, 2015, A1. Location in patent: Page/Page column 23
