475111-38-7
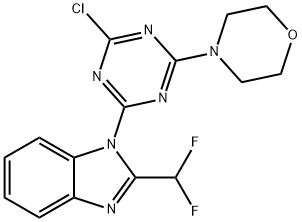 475111-38-7 结构式
475111-38-7 结构式
基本信息
4-(4-氯-6-(2-(二氟甲基)-1H-苯并[D]咪唑-1-基)-1,3,5-三嗪-2-基)吗啉
-1,3,5-triazin-2-yl)
-1H-benzo[d]imidazol-1-yl)
4-(4-Chloro-6-(2-(difluoromethyl)
Oxazole,2-(chloromethyl)-8-methyl-
Ethanone,2-bromo-1-[7-(trifluoromethyl)phenyl]-
1-[4-chloro-6-(Morpholin-4-yl)-1,3,5-triazin-2-yl]-2-(difluoroMethyl)-1H-1,3-be
1-(4-chloro-6-Morpholino-1,3,5-triazin-2-yl)-2-(difluoroMethyl)-1H-benzo[d]iMidazole
1-[4-chloro-6-(4-morpholinyl)-1,3,5-triazin-2-yl]-2-(difluoromethyl)-1H-Benzimidazole
1H-Benzimidazole, 1-[4-chloro-6-(4-morpholinyl)-1,3,5-triazin-2-yl]-2-(difluoromethyl)-
物理化学性质
制备方法

6601-22-5

705-09-9
![4-(4-氯-6-(2-(二氟甲基)-1H-苯并[D]咪唑-1-基)-1,3,5-三嗪-2-基)吗啉](/CAS/GIF/475111-38-7.gif)
475111-38-7
以2-(二氟甲基)-1H-苯并[d]咪唑(67.0g,400mmol)和2,4-二氯-6-吗啉代-1,3,5-三嗪(94.0g,400mmol)为原料,在无水碳酸钾(221.1g,1600mmol)的存在下,于DMF(1.60L)中室温搅拌反应4小时。反应完成后,将混合物倒入水中,析出白色沉淀。通过过滤收集沉淀,并依次用DMF和丙酮洗涤。随后,将固体在减压下干燥,得到白色结晶产物4-(4-氯-6-(2-(二氟甲基)-1H-苯并[d]咪唑-1-基)-1,3,5-三嗪-2-基)吗啉(131.5g,358.6mmol),收率为90%。质谱分析显示m/z:366(M+)。1H-NMR(CDCl3)数据如下:δ 3.80-3.87(4H,m),3.94-4.01(4H,m),7.38-7.53(2H,m),7.58(1H,t,J=54Hz),7.90(1H,d,J=7Hz),8.42(1H,d,J=7Hz)。
参考文献:
[1] Patent: US2007/244110, 2007, A1. Location in patent: Page/Page column 4
[2] Patent: WO2014/164942, 2014, A1. Location in patent: Paragraph 0206-0207
[3] ACS Medicinal Chemistry Letters, 2013, vol. 4, # 2, p. 206 - 210
[4] Patent: WO2014/5182, 2014, A1. Location in patent: Page/Page column 40
[5] Patent: WO2011/114275, 2011, A1. Location in patent: Page/Page column 74
