476004-81-6
 476004-81-6 结构式
476004-81-6 结构式
基本信息
吲哚-2-硼酸嚬哪醇酯
吲哚-2-硼酸频哪醇酯
1H-吲哚-2-硼酸频哪醇酯
(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)-1H-吲哚
2-(4,4,5,5-四甲基-1,3,2-二氧杂硼烷-2-基)-1H-吲哚
2-(4,4,5,5-四甲基-1,3,2-二氧杂硼杂环戊烷-2-基)-1H-吲哚
2-Indoleboronic acid pinacol ester
Indole-2-boronic acid pinacol ester
1H-Indole-2-boronic acid picol ester
1H-Indole-2-boronic acid pinacol ester
Indole-2-boronic acid pinacol ester 95%
2-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indole
1H-Indole, 2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-
2-(((1H-indol-2-yl)boryl)oxy)-3-hydroxy-2,3-dimethylbutanoate
2-((1H-Indol-2-yl)boryloxy)-3-hydroxy-2,3-dimethylbutanoic acid
物理化学性质
制备方法
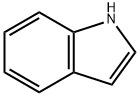
120-72-9
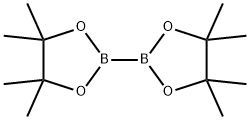
73183-34-3

476004-81-6
以吲哚和联硼酸频那醇酯为原料合成吲哚-2-硼酸频哪醇酯的一般步骤:在氩气保护下,向干燥的圆底烧瓶中加入二甲基(1,5-环辛二烯)二铱(I) (64 mg, 0.096 mmol)和4,4'-二叔丁基-2,2'-联吡啶(52 mg, 0.19 mmol)。将烧瓶抽真空并用氩气回填三次。随后加入吲哚(1.50 g, 12.8 mmol)和双(频哪醇合)二硼(1.62 g, 6.40 mmol),再次用氩气吹扫烧瓶。注入无水己烷(38.5 mL),将反应混合物在室温下氩气氛围中搅拌8小时。反应完成后,减压浓缩反应混合物,所得残余物通过硅胶柱色谱纯化(洗脱剂:0-10%乙酸乙酯/己烷),得到吲哚-2-硼酸频哪醇酯(2.66 g, 85%收率)。产物经1H NMR (300 MHz, d6-DMSO)表征:δ 11.27 (s, 1H), 7.55 (d, J = 7.9 Hz, 1H), 7.38 (dd, J = 8.2, 0.8 Hz, 1H), 7.15-7.06 (m, 1H), 7.00-6.92 (m, 1H), 6.90-6.87 (m, 1H), 1.32 (s, 12H)。
参考文献:
[1] Journal of the American Chemical Society, 2015, vol. 137, # 7, p. 2665 - 2673
[2] Patent: WO2014/22744, 2014, A1. Location in patent: Paragraph 0117
[3] Journal of the American Chemical Society, 2014, vol. 136, # 18, p. 6566 - 6569
[4] Dalton Transactions, 2015, vol. 44, # 29, p. 13007 - 13016
[5] Organic Letters, 2012, vol. 14, # 16, p. 4266 - 4269
