521284-19-5
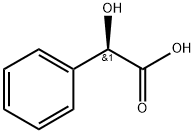
 521284-19-5 结构式
521284-19-5 结构式
基本信息
米拉贝隆中间体1
(R)-2-羟基-N-(4-硝基苯乙基)-2-苯基乙酰胺
R) -羟基-N-[2-(4-硝基苯基)乙基]-苯乙酰胺
(R)-2-羟基-N-[2-(4-硝基苯基)乙基]苯乙酰胺
(R)-2-羟基-N-[2-(4-硝基苯基)乙基]-2-苯乙酰胺
R-(ALPHA)-羟基-N-[2-(4-硝基苯基)乙基]苯乙酰胺
(ALPHAR)-ALPHA-羟基-N-[2-(4-硝基苯基)乙基]苯乙酰胺
(R)-2-羟基-N-(4-硝基苯乙基)-2-苯基乙酰胺 (米拉贝隆中间体1
(ALPHAR)-ALPHA-羟基-N-[2-(4-硝基苯基)乙基]苯乙酰胺(中间体1)
(R)-2-Hydroxy-N-(4-nitrophenethyl)-2-phenylacetamide
(R)-N-(4-nitrophenethyl)-2-hydroxy-2-phenylacetamide
Mirabegron Impurity 8 (Mirabegron stage -I Impurity )
R-a-hydroxy-N-[2-(4-nitrophenyl)ethyl]- BenzeneacetaMide
(R) -N- (4-Nitrophenylethyl) -2-hydroxy-2-phenylacetamide
(2R)-2-Hydroxy-N-[2-(4-nitrophenyl)ethyl]-2-phenylacetamide
BenzeneacetaMide, -hydroxy-N-[2-(4-nitrophenyl)ethyl]-, (R)-
R-alpha-hydroxy-N-[2-(4-nitrophenyl)ethyl]- BenzeneacetaMide
R-2-((4-aminophenethyl)amino)-1-phenylethan-1-ol hydrochloride
物理化学性质
制备方法
611-71-2

29968-78-3
![(ALPHAR)-ALPHA-羟基-N-[2-(4-硝基苯基)乙基]苯乙酰胺](/CAS/GIF/521284-19-5.gif)
521284-19-5
一般步骤:向搅拌的(R)-扁桃酸(97.61 g)的乙腈(1000 mL)溶液中,于28℃(±2℃)下缓慢加入硼酸三甲酯(66.66 g)。加毕,将反应体系升温至58℃(±2℃)并维持90分钟。保持此温度,向反应液中加入4-硝基苯乙胺盐酸盐(100.0 g)。随后,缓慢滴加N,N-二异丙基乙胺(82.92 g),并将反应混合物加热回流12小时。反应完成后,将体系冷却至30℃(±2℃),用乙酸乙酯(1100 mL)和1M盐酸水溶液(1400 mL)稀释。分离有机相,依次用1M盐酸水溶液、5%氢氧化钠水溶液及饱和食盐水洗涤。有机相经减压浓缩后,残余物用甲苯(500 mL)重结晶。所得固体经甲苯洗涤,于48℃(±2℃)下真空干燥,得到浅黄色晶体状产物(R)-2-羟基-N-[2-(4-硝基苯基)乙基]-2-苯基乙酰胺。产量:125 g(84.3%);HPLC纯度:98.65%。
参考文献:
[1] Advanced Synthesis and Catalysis, 2017, vol. 359, # 20, p. 3665 - 3673
[2] Patent: WO2015/44965, 2015, A1. Location in patent: Page/Page column 32
[3] Patent: CN105481705, 2016, A. Location in patent: Paragraph 0017; 0018; 0019
[4] Patent: EP1440969, 2004, A1. Location in patent: Page 5-6
[5] Patent: EP1559427, 2005, A1. Location in patent: Page/Page column 5-6
