5395-50-6
 5395-50-6 结构式
5395-50-6 结构式
基本信息
1,3,4,6-四羟甲基甘脲
四羟甲基咪唑杂[4,5-D]咪唑-2,5[1H,2H]-二酮
四氢-1,3,4,6-四(羟甲基)咪唑并[4,5-D]咪唑-2,5(1H,3H)-二酮
Tetramethylol acetylenediurea
Tetramethylol acetylene diuriene
Tetrakis (hydroxy methy1) glycoluril
1,3,4,6-tetramethylol-3a,6a-dihydroimidazo[4,5-d]imidazole-2,5-quinone
1,3,4,6-Tetrakis(hydroxymethyl)octahydroimidazo[4,5-d]imidazole-2,5-dione
1,3,4,6-Tetrakis-hydroxymethyl-tetrahydro-imidazo(4,5-d)imidazole-2,5-dione
1,3,4,6-tetrakis(hydroxymethyl)-3a,6a-dihydroimidazo[4,5-d]imidazole-2,5-dione
Tetrahydro-1,3,4,6-tetrakis(hydroxymethyl)imidazo[4,5-d]imidazol-2,5(1H,3H)-dion
1,3,4,6-Tetrakis(hydroxyMethyl)tetrahydroiMidazo[4,5-d]iMidazole-2,5(1H,3H)-dione
物理化学性质
制备方法
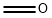
50-00-0

496-46-8

5395-50-6
在配备有机械搅拌器、温度计和回流冷凝器的合适反应容器中,加入688份(10mol)44%甲醛水溶液,并用22份0.5N NaOH溶液调节pH至8.7。将反应体系温度维持在40℃,随后向其中加入284份(2mol)甘脲。反应过程中,温度逐渐升至55℃,此时大部分甘脲溶解。约15分钟后,用5份0.5N NaOH溶液将反应体系的pH调节至8.0,得到澄清的浅黄色溶液。将此溶液在50℃下进行减压蒸馏,除去水分,直至反应容器内剩余物约为640份。将浓缩后的糖浆状产物倒入800份甲醇中,析出白色结晶沉淀。经过滤和干燥后,得到483份(收率92%)1,3,4,6-四(羟甲基)四氢咪唑并[4,5-d]咪唑-2,5(1H,3H)-二酮,熔点为132℃~136℃。
参考文献:
[1] Patent: WO2014/166347, 2014, A1. Location in patent: Page/Page column 14
[2] Patent: WO2015/143974, 2015, A1. Location in patent: Page/Page column 15; 16
[3] Chemistry of Heterocyclic Compounds, 2006, vol. 42, # 3, p. 365 - 376
[4] Russian Journal of Applied Chemistry, 2016, vol. 89, # 1, p. 132 - 139
[5] Zh. Prikl. Khim. (S.-Peterburg, Russ. Fed.), 2016, vol. 89, # 1, p. 103 - 111,9
