59279-58-2
 59279-58-2 结构式
59279-58-2 结构式
基本信息
BOC-L-谷氨酸(卞酯)-1-甲酯
BOC-L-谷氨酸5苄酯ALPHA甲酯
叔丁氧羰基-L-谷氨酸 5-苄基 1-甲基酯
N-叔丁氧羰基-5-苄基-L-谷氨酸 -1-甲酯
(S)-5-苄基-2 -((叔丁氧基羰基)氨基)戊二酸甲酯
BOC-L-GLUTAMIC ACID Γ-BENZYL Α-METHYL ESTER
Boc-L-Glu(OBzl)-OMe
(Tert-Butoxy)Carbonyl Glu(OBzl)-OMe
Boc-L-glutamicacid-benzyla-methylester
Boc-L-Glutamic Acid 5-Benzyl 1-Methyl Ester
Boc-L-glutamic acid γ-benzyl α-methyl ester
BOC-L-GLUTAMIC ACID-Y-BENZYL A-METHYL-DIESTER
BOC-L-GLUTAMIC ACID-GAMMA-BENZYL ALPHA-METHYL-DIESTER
BOC-L-GLUTAMIC ACID .GAMMA.-BENZYL .ALPHA.-METHYL ESTER
BOC-L-GLUTAMIC ACID GAMMA-BENZYL ESTER ALPHA-METHYL ESTER
物理化学性质
制备方法
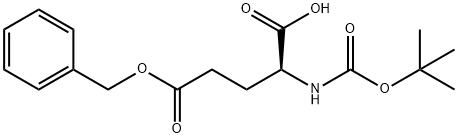
13574-13-5
74-88-4

59279-58-2
步骤1:将碘甲烷(1.01 mL,16.3 mmol)缓慢滴加到(2S)-5-(苄氧基)-2-{[(叔丁氧基)羰基]氨基}-5-氧代戊酸(5.00 g)的THF(14.82 mmol)和DMF(25 mL)混合溶液中,同时加入K2CO3(2.25 g,16.3 mmol)。反应混合物在室温下搅拌约3小时后,再次加入碘甲烷(1.01 mL,16.3 mmol)。随后,向反应混合物中加入EtOAc,并用10% Na2S2O3溶液洗涤三次,然后用MgSO4干燥。减压浓缩除去溶剂后,粗产物通过硅胶柱色谱(100:1,随后50:1 CHCl3/MeOH)纯化,得到(S)-5-苄基-2-((叔丁氧基羰基)氨基)戊二酸甲酯(4.20 g,12.23 mmol,收率82%)。ESIMS检测到C18H25NO6的m/z为352(M + H)。
参考文献:
[1] Journal of Medicinal Chemistry, 2016, vol. 59, # 5, p. 1899 - 1913
[2] Patent: US2008/318957, 2008, A1. Location in patent: Page/Page column 41-42
[3] Patent: WO2012/109164, 2012, A1. Location in patent: Page/Page column 39
[4] Organic and Biomolecular Chemistry, 2007, vol. 5, # 12, p. 1915 - 1923
[5] Organic Letters, 2016, vol. 18, # 9, p. 1968 - 1971
