5965-59-3
 5965-59-3 结构式
5965-59-3 结构式
基本信息
4-羟基-2-喹啉羧酸甲酯
Kynurenic acid, methyl ester
Methyl 4-hydroxyquinaldinate
Methyl 4-hydroxy-2-quinolinecarboxylate
Methyl 4-Hydroxyquinoline-2-carboxylate
methyl 4-oxo-1H-quinoline-2-carboxylate
4-oxo-1H-quinoline-2-carboxylic acid methyl ester
2-Quinolinecarboxylic acid, 4-hydroxy-, methyl ester
物理化学性质
制备方法
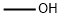
67-56-1
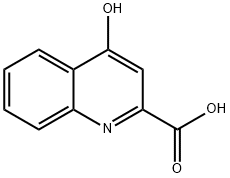
492-27-3

5965-59-3
将4-羟基喹啉-2-羧酸(500 mg, 2.65 mmol)悬浮于无水甲醇中,缓慢加入浓硫酸(96%,40滴)至溶液澄清。将反应混合物加热至回流,保持回流状态反应24小时(通过LC-MS监测反应进度,直至起始原料完全消失)。反应完成后,冷却至室温。使用旋转蒸发器除去溶剂,得到粗产物。将粗产物溶于甲醇(1 mL)中,加入去离子水(10 mL)稀释。随后,缓慢加入饱和碳酸氢钠溶液,直至白色沉淀析出。过滤收集沉淀,并用二乙醚洗涤,得到白色固体产物,收率65%(0.35 g)。产物经1H NMR(DMSO-d6, 500 MHz)和LC-MS(ES+)表征:1H NMR δ 3.52(s, 3H), 6.48(s, 1H), 7.25(t, 1H, J = 7.0 Hz), 7.58(t, 1H, J = 7.0 Hz), 7.96(d, 1H, J = 8.5 Hz), 8.05(d, 1H, J = 8.5 Hz), 11.32(s, 1H); LC-MS m/z 204.09 [M+H]+, 计算值204.06。
参考文献:
[1] Journal of Medicinal Chemistry, 2002, vol. 45, # 3, p. 559 - 562
[2] Bioorganic and Medicinal Chemistry, 2004, vol. 12, # 20, p. 5453 - 5463
[3] Patent: WO2012/6068, 2012, A2. Location in patent: Page/Page column 67-68
[4] Monatshefte fuer Chemie, 1921, vol. 42, p. 100
[5] Monatshefte fuer Chemie, 1922, vol. 43, p. 479
