61090-37-7
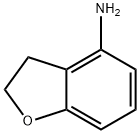 61090-37-7 结构式
61090-37-7 结构式
基本信息
4-氨基-2,3-二氢苯并呋喃
2,3-二氢-4-氨基苯并呋喃
4-Amino-2,3-dihydrobenzofuran
2,3-dihydrobenzofuran-4-aMine
2,3-DIHYDRO-4-BENZOFURANAMINE
4-Benzofuranamine, 2,3-dihydro-
2,3-Dihydro-1-benzofuran-4-amine
2,3-Dihydro-1-benzofuran-4-ylamine
5H,6H,7H,7aH-cyclopenta[b]pyran-5-aMine
2,3-dihydrobenzofuran-4-amine hydrochloride
物理化学性质
制备方法

76093-72-6

61090-37-7
4-氨基-2,3-二氢苯并呋喃(1c)的合成:将化合物1b(4.10 g,16.3 mmol)溶解于48%氢溴酸水溶液(20.0 mL)中,充分搅拌混合。将反应混合物于110°C下加热回流16小时。反应完成后,将混合物冷却至室温,随后在0°C冰浴条件下缓慢加入氢氧化钠颗粒,调节pH至约9。用乙酸乙酯(50 mL × 4)多次萃取反应混合物。合并有机相,用无水硫酸钠干燥,过滤。减压浓缩滤液,得到粗产物。通过中压柱色谱(Smart Flash EPCLC W-Prep 2XY系统,洗脱剂:正己烷/乙酸乙酯 = 1/1)纯化粗产物,得到4-氨基-2,3-二氢苯并呋喃(化合物1c)(1.49 g,11.0 mmol,收率67.7%),为无色固体。TLC Rf = 0.30(展开剂:正己烷/乙酸乙酯 = 1/1)。1H NMR(400 MHz,CDCl3)δ 6.94(dd,J = 8.4, 8.4 Hz,1H),6.28(dd,J = 0.4, 7.6 Hz,1H),6.23(dd,J = 0.4, 7.6 Hz,1H),4.59(t,J = 8.4 Hz,2H),3.60(br s,2H),3.02(t,J = 8.4 Hz,2H)。
参考文献:
[1] Patent: EP2881397, 2015, A1. Location in patent: Paragraph 0053; 0058; 0059
[2] Patent: US2016/303089, 2016, A1. Location in patent: Paragraph 0161; 0168; 0169; 0170; 0171
[3] Patent: US2008/318941, 2008, A1. Location in patent: Page/Page column 39
[4] Patent: WO2009/23844, 2009, A2. Location in patent: Page/Page column 127-128
[5] Patent: US2008/200471, 2008, A1. Location in patent: Page/Page column 60; 61-62
