62718-28-9
 62718-28-9 结构式
62718-28-9 结构式
基本信息
1-甲基哌啶-4-羧酰胺
1-甲基异3-哌啶甲酰胺
1-METHYLPIPERIDINE-4-CARBOXAMIDE
N-METHYLPIPERIDINE-4-CARBOXAMIDE
1-Methyl-4-PiperidinecarboxaMide
4-Piperidinecarboxamide, 1-methyl-
4-Piperidinecarboxamide,1-methyl-(9CI)
物理化学性质
制备方法

39546-32-2
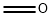
50-00-0

62718-28-9
将哌啶-4-甲酰胺(10 g,78 mmol)溶于蒸馏水(100 mL)中,加入37%甲醛水溶液(7.6 mL,相当于2.81 g HCHO,93.6 mmol)。在氩气保护下,加入湿的10% Pd/C催化剂(8刮刀勺),并将混合物在25℃和50 psi氢气压力下氢化43小时。反应完成后,通过硅藻土过滤除去催化剂,催化剂用水和甲醇洗涤。合并滤液,蒸发至干。残余物通过硅胶柱色谱(60×5 cm)纯化,使用8%-10%-20%(10%浓氨水的甲醇溶液)-二氯甲烷作为洗脱液,得到1-甲基哌啶-4-羧酰胺(7.15 g,产率64%)。产物表征数据如下:FABMS:m/z 143.1(MH+);HRFABMS:m/z 143.1184(MH+),计算值C7H15N2O:m/z 143.1184;1H NMR(d6-DMSO)δ 1.50-1.57(4H,m,CH2),1.76-1.94(4H,m,CH2),2.10(3H,s,-NCH3),2.72(1H,m,CH),6.68-7.18(2H,m,CONH2);13C NMR(d6-DMSO)δ 41.2(CH3),28.5, 28.5, 54.9, 54.9(CH2),46.2(CH),176.7(C)。
参考文献:
[1] Journal of Medicinal Chemistry, 2003, vol. 46, # 7, p. 1116 - 1119
[2] Patent: WO2004/22561, 2004, A1. Location in patent: Page 110
[3] Journal of Medicinal Chemistry, 2005, vol. 48, # 24, p. 7520 - 7534
[4] Helvetica Chimica Acta, 1954, vol. 37, p. 1672,1676

