69651-48-5
 69651-48-5 结构式
69651-48-5 结构式
基本信息
(S)-2-((叔丁氧基羰基)氨基)-2-(4-羟基苯基)乙酸
(S)-2-((叔-丁氧羰基)氨基)-2-(4-羟基苯基)乙酸
N-Boc-S-4-Hydroxyphenylglycine
N-Boc-4-hydroxyphenyl-l-glycine
Boc-(S)-2-amino-2-(4-hydroxyphenyl)acetic acid
(S)-2-((tert-Butoxycarbonyl)amino)-2-(4-hydroxyphenyl)acetic acid
Benzeneacetic acid,a-[[(1,1-dimethylethoxy)carbonyl]amino]-4-hydroxy-, (aS)-
Benzeneacetic acid, α-[[(1,1-dimethylethoxy)carbonyl]amino]-4-hydroxy-, (αS)-
(2S)-2-(4-hydroxyphenyl)-2-[(2-methylpropan-2-yl)oxycarbonylamino]acetic acid
制备方法
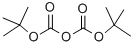
24424-99-5

32462-30-9

69651-48-5
一般步骤:在0℃条件下,将L-(+)-对羟基苯甘氨酸(1.00 g,5.98 mmol)和碳酸氢钠(2.50 g,29.11 mmol)溶解于1,4-二恶烷(20 mL)和水(20 mL)的混合溶剂中。缓慢加入二碳酸二叔丁酯(1.40 g,6.58 mmol),随后将反应混合物升温至室温并搅拌18小时。反应完成后,减压蒸发除去有机溶剂,将残余物用乙酸乙酯(300 mL)和0.5 M盐酸水溶液(50 mL)进行液-液分配。分离有机相,依次用0.5 M盐酸水溶液、水和饱和食盐水洗涤,无水硫酸钠干燥,过滤后减压浓缩,得到白色固体产物(S)-2-((叔丁氧基羰基)氨基)-2-(4-羟基苯基)乙酸(1.6 g,定量收率)。产物经1H NMR(400 MHz,CDCl3)表征:δ 8.80(宽单峰,1H),7.12(双峰,2H),6.62(双峰,2H),5.80(双峰,1H),5.00(双峰,1H),1.22(单峰,9H)。质谱(EI)分析结果与分子式C11H17NO5相符:[M-H]- m/z 266。
参考文献:
[1] Synlett, 2008, # 15, p. 2355 - 2359
[2] Patent: WO2009/55077, 2009, A1. Location in patent: Page/Page column 410
[3] Chemistry - A European Journal, 2010, vol. 16, # 34, p. 10523 - 10534
[4] Patent: WO2014/210436, 2014, A2. Location in patent: Paragraph 00411; 00412
[5] Advanced Synthesis and Catalysis, 2017, vol. 359, # 17, p. 2897 - 2900
