7400-06-8
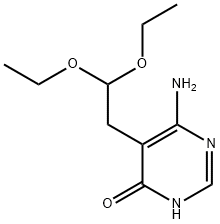 7400-06-8 结构式
7400-06-8 结构式
基本信息
6-氨基-5-(2,2-二乙氧基乙基)-4-羟基嘧啶
6-氨基-5-(2,2-二乙氧基乙基)嘧啶-4(3H)-酮
6-氨基-5-(2,2-二乙氧基乙基)-4(3H)-嘧啶酮
Tofacitinib Impurity 203
6-Amino-5-(2,2-diethoxyethyl)
6-Amino-5-(2,2-diethoxyethyl)pyrimidin-4-ol
6-amino-5-(2,2-diethoxyethyl)-1H-pyrimidin-4-one
6-AMino-5-(2,2-diethoxyethyl)-4(3H)-pyriMidinone
6-aMino-5-(2,2-diethoxyethyl)pyriMidin-4(3H)-one
4(3H)-PyriMidinone, 6-aMino-5-(2,2-diethoxyethyl)-
6-aMino-5-(2,2-diethoxyethyl)-1,4-dihydropyriMidin-4- one
物理化学性质
制备方法

7400-05-7

7400-06-8
步骤i: 将6-氨基-5-(2,2-二乙氧基乙基)-2-巯基-4-羟基嘧啶(11.6 mmol)[制备方法参见Davoll, J., J. Chem. Soc., 1960, pp131-138]溶解于水(150 mL)和氨水溶液(9 mL)的混合溶剂中,加热至90℃。向反应混合物中分批加入Raney Nickel悬浮液(每次2-3 mL),直至TLC和LC/MS分析显示反应完成。将反应混合物冷却至室温,通过硅藻土垫过滤,滤饼用水洗涤(2×25 mL)。合并的水相滤液经冷冻干燥,得到6-氨基-5-(2,2-二乙氧基乙基)-4-羟基嘧啶,为灰白色粉末,产量2.23 g(85%)。LC/MS: RT = 1.37 min; m/z = 182 [M-EtOH + H]+。总运行时间为3.75 min。1H NMR (DMSO-d6): δ 1.07 (t, 6H); 3.40 (m, 2H); 3.59 (m, 2H); 4.56 (t, 1H); 6.07 (br s, 2H); 7.70 (s, 1H); 11.43 (br s, 1H)。
参考文献:
[1] Patent: WO2007/104944, 2007, A1. Location in patent: Page/Page column 43
[2] Patent: WO2006/46023, 2006, A1. Location in patent: Page/Page column 123
[3] Patent: WO2008/75109, 2008, A1. Location in patent: Page/Page column 112
[4] Patent: WO2006/46024, 2006, A1. Location in patent: Page/Page column 118
[5] Patent: WO2007/125321, 2007, A2. Location in patent: Page/Page column 116
