77156-78-6
 77156-78-6 结构式
77156-78-6 结构式
基本信息
ETHYL 4-HYDROXY-6-METHOXYQUINOLINE-3-CARBOXYLATE
ETHYL 4-HYDROXY-6-METHOXY-3-QUINOLINECARBOXYLATE
ethyl 6-methoxy-4-oxo-1H-quinoline-3-carboxylate
ethyl 6-methoxy-4-hydroxy-3-quinoline-carboxylate
2-ethyl-6-methoxy-4-oxo-1H-quinoline-3-carboxylate
Ethyl 4-hydroxy-6-(methyloxy)-3-quinolinecarboxylate
Ethyl 6-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylate
Ethyl 1,4-dihydro-6-methoxy-4-oxoquinoline-3-carboxylate
4-Hydroxy-6-methoxy-3-quinolinecarboxylic acid ethyl ester
物理化学性质
制备方法
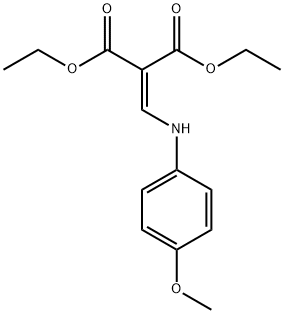
83507-70-4

77156-78-6
以2-[(4-甲氧基苯胺基)亚甲基]丙二酸二乙酯(CAS: 83507-70-4,71.4 g,0.24 mol)为原料,在150 mL二苯醚中制备溶液。将反应体系置于金属浴中,缓慢加热至243℃,并维持此温度反应3小时。反应过程中,蒸馏出15 mL液体。反应完成后,将混合物冷却至室温,随后加入250 mL己烷溶解。搅拌混合物3小时后,进行过滤操作。用己烷洗涤所得固体,干燥后得到4-羟基-6-甲氧基喹啉-3-羧酸乙酯(25.0 g,0.10 mol,产率41%),其纯度满足直接用于后续反应的要求。产物结构经1H-NMR(CDCl3)和LC-MS确认:1H-NMR(CDCl3)δ= 1.28(t,3H),3.85(s,3H),4.21(q,2H),7.34(dd,1H),7.55-7.62(m,2H),8.49(s,1H);LC-MS:Rt = 1.17 min;MS:m/z = 248 [M + 1]+。
参考文献:
[1] Patent: WO2006/2047, 2006, A2. Location in patent: Page/Page column 60
[2] European Journal of Medicinal Chemistry, 2015, vol. 103, p. 1 - 16
[3] Organic Process Research and Development, 2014, vol. 18, # 11, p. 1482 - 1491
[4] Tetrahedron Letters, 2017, vol. 58, # 8, p. 794 - 796
[5] Journal of the American Chemical Society, 1946, vol. 68, p. 1204,1206
