83741-35-9
 83741-35-9 结构式
83741-35-9 结构式
基本信息
4-溴-1H-苯并咪唑
4-溴-1H-苯并[D]咪唑
7 - 溴-1H -苯并[D]咪唑
4-Bromo-1H-benzimidazole
7-Bromo-1H-benzimidazole
7-Bromo-1H-benzoimidazole
1H-BenziMidazole,7-broMo-
4-broMo-1H-1,3-benzodiazole
4-Bromo-1H-benzimidazole 98%
7-Bromo-1H-benzo[d]imidazole
4-bromo-1H-benzo[d]imidazole
1H-Benzimidazole,4-bromo-(9CI)
物理化学性质
制备方法
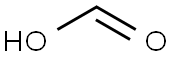
64-18-6

1575-36-6

83741-35-9
步骤2:4-溴-1H-苯并咪唑的合成; 将1,2-二氨基-3-溴苯(2.00g,10.7mmol)溶解于甲酸(10mL)中,于100℃下搅拌反应1小时。反应完成后,用4M氢氧化钠溶液调节反应混合物的pH至14,促使产物以固体形式析出。通过过滤分离固体产物,用水洗涤后风干,得到灰白色固体产物。将滤液在室温下静置数日,可析出另一批纯度相同的产物。总产量为1.98克,产率94%。产物经1H NMR(500MHz,ds-DMSO)表征:δ12.83(1H,s),8.30(1H,s),7.58(1H,d,J = 7.9Hz),7.41(1H,d,J = 7.1 Hz),7.14(1 H,t,J = 7.8 Hz);质谱(ES+)m/z:197,199 [MH+]。
参考文献:
[1] Patent: WO2016/161145, 2016, A1. Location in patent: Paragraph 00697
[2] Patent: WO2013/26914, 2013, A1. Location in patent: Page/Page column 108
[3] Patent: WO2006/59149, 2006, A1. Location in patent: Page/Page column 41
[4] Patent: WO2006/97766, 2006, A1. Location in patent: Page/Page column 23
[5] Patent: WO2006/21805, 2006, A1. Location in patent: Page/Page column 76
