852471-15-9
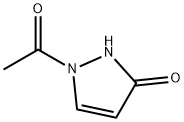 852471-15-9 结构式
852471-15-9 结构式
基本信息
1-乙酰基-3-羟基吡唑
1-乙酰基-1H-吡唑-3(2H)-酮
1-(3-羟基-1H-吡唑-1-基)乙酮
1-乙酰基1,2-二氢-3H-吡唑-3-酮
1-Acetyl-1H-pyrazol-3(2H)-one
1-Acetyl1,2-dihydro-3H-pyrazole-3-one
1-Acetyl-1,2-dihydro-3h-pyrazol-3-one
1-(3-hydroxy-1H-pyrazol-1-yl)ethanone
1-acetyl-2,3-dihydro-1H-pyrazol-3-one
3H-Pyrazol-3-one, 1-acetyl-1,2-dihydro-
1-neneneba acetyl 1,2-dihydro-3H-pyrazole-3-one
制备方法
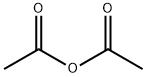
108-24-7

137-45-1

852471-15-9
向配备有磁力搅拌子和回流冷凝管的100 mL圆底烧瓶中,依次加入1,2-二氢-3H-吡唑-3-酮(4.97 g,59.11 mmol)和吡啶(25 mL,309.1 mmol)。将反应混合物加热至95℃并持续搅拌。在3分钟内,缓慢滴加由乙酸酐(5.6 mL,59.35 mmol)溶解于吡啶(10 mL,123.6 mmol)中的溶液。滴加完毕后,继续在95℃下搅拌反应混合物3小时。反应完成后,通过减压蒸馏移除溶剂。所得固体残留物用40 mL乙醚进行研磨,随后过滤,并用乙醚洗涤,最终干燥得到1-乙酰基-1H-吡唑-3(2H)-酮(6.96 g,产率93%)。产物经1H NMR(400 MHz,DMSO-d6)表征:δ 10.96(s,1H),8.13(d,J = 3.0 Hz,1H),6.01(d,J = 3.0 Hz,1H),2.48(s,3H)。
参考文献:
[1] Patent: WO2018/64632, 2018, A1. Location in patent: Paragraph 00650; 00651
[2] Patent: WO2011/26937, 2011, A1. Location in patent: Page/Page column 26
[3] ChemMedChem, 2015, vol. 10, # 7, p. 1184 - 1199
[4] Patent: US2005/165005, 2005, A1. Location in patent: Page/Page column 44
[5] Patent: WO2013/162072, 2013, A1. Location in patent: Page/Page column 960