860457-92-7
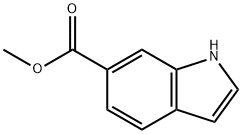
 860457-92-7 结构式
860457-92-7 结构式
基本信息
3-溴吲哚-6-甲酸甲酯
3-溴-1H-吲哚-6-羧酸甲酯
1H-吲哚-6-羧酸,3-溴-,甲酯
Methyl 3-broMo-1H-indole-6-carboxylate
3-Bromo-1H-indole-6-carboxylic acid methyl ester
1H-Indole-6-carboxylic acid, 3-bromo-, methyl ester
物理化学性质
制备方法
50820-65-0

860457-92-7
在氮气气氛下,将1H-吲哚-6-羧酸甲酯(1.00g,5.71mmol)溶解于无水DMF(10mL)中。在-60℃下,缓慢滴加N-溴代琥珀酰亚胺(NBS,1.04g,5.84mmol)的无水DMF(10mL)溶液,滴加时间超过20分钟。反应混合物在-60℃下搅拌,随后在3小时内缓慢升温至室温。反应完成后,用乙酸乙酯(100mL)和水(20mL,重复三次)萃取。合并有机层,真空浓缩除去溶剂。残余物通过硅胶柱色谱纯化,洗脱剂为石油醚和乙酸乙酯(乙酸乙酯比例从0%开始梯度增加),得到3-溴吲哚-6-甲酸甲酯(1.41g,收率98%)。产物经1H NMR(600MHz,CD3OD)表征:δ8.15(s,1H),7.80(d,J = 6.0Hz,1H),7.50(m,2H),3.94(s,3H)。
参考文献:
[1] Patent: WO2017/197240, 2017, A1. Location in patent: Page/Page column 204
[2] Patent: WO2009/147121, 2009, A1. Location in patent: Page/Page column 35
[3] Advanced Synthesis and Catalysis, 2018, vol. 360, # 4, p. 626 - 630
[4] Patent: US2005/165049, 2005, A1. Location in patent: Page/Page column 15
[5] Patent: WO2017/184658, 2017, A1. Location in patent: Page/Page column 19; 20