870119-58-7
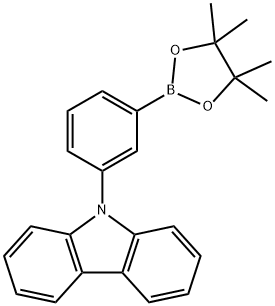 870119-58-7 结构式
870119-58-7 结构式
基本信息
9-[3-(硼酸频哪醇酯)苯基]咔唑
(3-(咔唑-9-基)苯基)频哪醇硼酸酯
(3-(咔唑-9-基)苯基)频哪醇硼酸酯 10G
9-(3-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)苯基)咔唑
9-[3-(4,4,5,5-四甲基-1,3,2-二氧杂环戊硼烷-2-基)苯基]咔唑
(3-(carbazole-9H)Phenyl)Picol ester
(3-(carbazole-9H)Phenyl)Pinacol ester
(3-(Carbazole-9H)Phenyl)Pinacol Ester,>98%
3-(9H-Carbazol-9-yl)phenylboronic acid pinacol ester
(3-(carbazole-9H)Phenyl)Pinacol ester ISO 9001:2015 REACH
9-(3-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)carbazole
2-[3-(9H-Carbazol-9-yl)phenyl]-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
9-[3-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-9H-carbazole
9H-Carbazole,9-[3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-
物理化学性质
制备方法

185112-61-2
73183-34-3

870119-58-7
在氮气保护下,将9-(3-溴苯基)-9H-咔唑(10.5 g, 1.0 equiv)、联硼酸频那醇酯(9.93 g, 1.2 equiv)、乙酸钾(10.9 g, 3.0 equiv)和无水二甲基亚砜(190 mL)加入反应瓶中,加热至60℃并搅拌15分钟。随后,加入[1,1'-双(二苯基膦基)二茂铁]二氯化钯(II)二氯甲烷络合物(0.799 g, 0.03 equiv)。将反应混合物加热至80℃,持续搅拌反应9小时。反应完成后,冷却至室温。向反应液中加入水(250 mL)和甲苯(500 mL),搅拌混合。分离水层,用甲苯对水层进行两次萃取。合并有机层,加入硫酸镁和活性粘土进行干燥。过滤除去干燥剂,减压浓缩除去甲苯。所得固体用冷甲醇洗涤,减压干燥,得到目标产物9-(3-(4,4,5,5-四甲基-1,3,2-二氧杂硼杂环戊烷-2-基)苯基)-9H-咔唑(9.86 g, 产率80%),为白色晶体。
参考文献:
[1] Patent: EP1820801, 2007, A1. Location in patent: Page/Page column 83
[2] Patent: CN103951621, 2016, B. Location in patent: Paragraph 0072; 0073; 0074; 0077; 0078
[3] Patent: US2013/75706, 2013, A1. Location in patent: Paragraph 0075
[4] Patent: KR101622443, 2016, B1. Location in patent: Paragraph 0107; 0108; 0111; 0112
[5] Patent: US9379336, 2016, B2. Location in patent: Page/Page column 41; 42
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW0287011958703 | (3-(咔唑-9-基)苯基)频哪醇硼酸酯 | 870119-58-7 | 10G | 141元 |
| 2025/12/22 | T3386 | 9-[3-(4,4,5,5-四甲基-1,3,2-二氧杂环戊硼烷-2-基)苯基]咔唑 9-[3-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]carbazole | 870119-58-7 | 1g | 160元 |
| 2025/12/22 | XW0287011958702 | (3-(咔唑-9-基)苯基)频哪醇硼酸酯 | 870119-58-7 | 5G | 71元 |
