883500-66-1
 883500-66-1 结构式
883500-66-1 结构式
基本信息
4-溴-7-氟-1H-吲哚
1H-INDOLE,4-BROMO-7-FLUORO
4-BROMO-7-FLUORO-1H-INDOLE
物理化学性质
制备方法

364-73-8
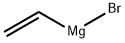
1826-67-1

883500-66-1
在-40℃下,将乙烯基溴化镁(300 mL的1.0 M THF溶液,300 mmol)缓慢滴加到4-溴-1-氟-2-硝基苯(22 g,100 mmol)的无水THF(700 mL)溶液中。保持反应温度在-40℃,反应1小时后,用饱和NH4Cl水溶液淬灭反应。随后,用EtOAc(乙酸乙酯)萃取反应混合物。合并有机层,用无水Na2SO4干燥,过滤后减压浓缩,得到粗产物。粗产物通过硅胶柱色谱法纯化,以适当比例的洗脱剂洗脱,得到目标化合物4-溴-7-氟-1H-吲哚(5 g,收率23.3%)。产物结构经1H NMR(CDCl3,400 MHz)确认:δ 6.56-6.58(m,1H),6.72-6.79(m,1H),7.06-7.17(m,1H),7.20-7.24(m,1H),8.45(s,1H)。
参考文献:
[1] European Journal of Organic Chemistry, 2006, # 13, p. 2956 - 2969
[2] Patent: WO2013/117522, 2013, A1. Location in patent: Page/Page column 39
[3] Patent: US2015/18367, 2015, A1. Location in patent: Paragraph 0173; 0174
[4] Patent: EP2570125, 2013, A1. Location in patent: Paragraph 0151
[5] Patent: WO2013/37960, 2013, A1. Location in patent: Page/Page column 74
