885618-31-5
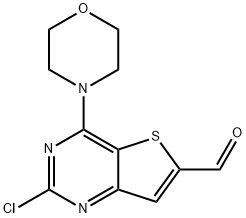 885618-31-5 结构式
885618-31-5 结构式
基本信息
2-氯-4-(4-吗啉)-噻吩并[3,2-D]嘧啶-6-羧醛
2-氯-4-(4-吗啉基)-噻吩并[3,2-D]嘧啶-6-甲醛
2-CHLORO-4-MORPHOLINOTHIENO[3,2-D]PYRIMIDINE-6-CARBALDEHYDE
2-CHLORO-4-MORPHOLINOTHIENO[3,2-D]PYRIMIDINE-6-CARBALDEHYDE-5
2-chloro-4-(Morpholin-4-yl)thieno[3,2-d]pyriMidine-6-carbaldehyde
2-chloro-4-(4-morpholinyl)-Thieno[3,2-d]pyrimidine-6-carboxaldehyde
2-Chloro-4-(Morpholin-4-yl)thieno[3,2-d]pyriMidine-6-carboxaldehyde
Thieno[3,2-d]pyriMidine-6-carboxaldehyde, 2-chloro-4-(4-Morpholinyl)-
物理化学性质
制备方法

68-12-2
![2-氯-4-(4-吗啉)-噻吩并[3,2-D]嘧啶-6-羧醛](/CAS/GIF/885618-31-5.gif)
885618-31-5
以N,N-二甲基甲酰胺为原料合成2-氯-4-(4-吗啉)-噻吩并[3,2-d]嘧啶-6-羧醛的一般步骤如下:参考实施例3,将2-氯-4-吗啉-4-基-噻吩并[3,2-d]嘧啶(65)(1.75 g,6.85 mmol)的无水THF(40 mL)溶液在-78℃下搅拌,缓慢加入2.5 M正丁基锂的己烷溶液(3.3 mL,1.2当量)。反应混合物在-78℃下继续搅拌1小时后,加入无水N,N-二甲基甲酰胺(796 μL,1.5当量)。保持-78℃反应1小时后,缓慢升温至室温。室温下继续搅拌2小时,反应完成后,将混合物倒入冰/水中,析出黄色沉淀。通过过滤收集沉淀,空气干燥,得到目标产物2-氯-4-(4-吗啉)-噻吩并[3,2-d]嘧啶-6-羧醛(1.50 g,收率77%)。产物经1H NMR(400 MHz,DMSO-d6)表征:δ 3.76(4H,t,J = 4.9 Hz),3.95(4H,t,J = 4.9 Hz),8.28(1H,s),10.20(1H,s)。
参考文献:
[1] Patent: WO2006/46031, 2006, A1. Location in patent: Page/Page column 30
[2] Patent: US2008/76768, 2008, A1. Location in patent: Page/Page column 7
[3] Patent: US2008/76758, 2008, A1. Location in patent: Page/Page column 73
[4] Patent: WO2007/122410, 2007, A1. Location in patent: Page/Page column 63
[5] Patent: WO2007/127183, 2007, A1. Location in patent: Page/Page column 136
