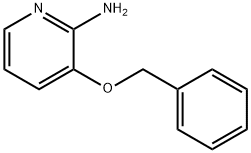
96428-50-1
 96428-50-1 结构式
96428-50-1 结构式
基本信息
8-苯甲氧基-2-甲基咪唑[1,2-A]并吡啶-3-羧酸乙酯
2-甲基-8-(苄氧基)咪唑并[1,2-A]吡啶-3-甲酸乙酯
2-甲基-8-(苯基甲氧基)咪唑并[1,2-A]吡啶-3-羧酸乙酯
咪唑并[1,2-A]吡啶-3-羧酸2-甲基-8-(苯基甲氧基)-乙酯
Imidazo[1,2-a]pyridine-3-carboxylicacid, 2-methyl-8-(phenylm...
Ethyl 8-(Benzyloxy)-2-MethyliMidazo[1,2-a]pyridine-3-carboxylate
Ethyl 8-(benzyloxy)-2-MethylH-iMidazo[1,2-a]pyridine-3-carboxylate
ethyl 2-methyl-8-phenylmethoxyimidazo[1,2-a]pyridine-3-carboxylate
8-BENZYLOXY-2-METHYL-IMIDAZO[1,2-A]PYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER
2-Methyl-8-(phenylmethoxy)imidazo[1,2-a]pyridine-3-carboxylic acid ethyl ester
Imidazo[1,2-a]pyridine-3-carboxylic acid, 2-methyl-8-(phenylmethoxy)-, ethyl ester
制备方法
24016-03-3

609-15-4
![2-甲基-8-(苄氧基)咪唑并[1,2-A]吡啶-3-甲酸乙酯](/CAS/GIF/96428-50-1.gif)
96428-50-1
实施例1A 8-(苄氧基)-2-甲基咪唑并[1,2-a]吡啶-3-羧酸乙酯的合成:将25g(124.8mmol)2-氨基-3-苄氧基吡啶溶解于781ml无水乙醇中,随后加入102.7g(624.2mmol)2-氯乙酰乙酸乙酯和2汤匙4A分子筛。将反应混合物加热至回流(油浴温度100℃),持续反应2天。反应完成后,将混合物浓缩,并在干冰冷却条件下,通过旋转蒸发器去除过量的2-氯乙酰乙酸乙酯。残余物通过硅胶柱色谱法纯化,流动相为环己烷:乙酸乙酯(梯度洗脱,比例从9:1至4:1)。最终得到20.81g目标化合物2-甲基-8-(苄氧基)咪唑并[1,2-a]吡啶-3-甲酸乙酯,收率为54%,纯度为99%。产物经LC-MS(方法2)分析:保留时间Rt = 1.12min,质谱(ESpos):m/z = 311(M + H)+。1H NMR(400MHz,DMSO-d6)数据如下:δ= 1.35(t,3H),2.59(s,3H),4.34(q,2H),5.32(s,2H),7.01-7.09(m,2H),7.33-7.48(m,3H),7.52(d,2H),8.81-8.86(m,1H)。
参考文献:
[1] Patent: US2014/128386, 2014, A1. Location in patent: Paragraph 0984; 0985; 0986; 0987; 0988
[2] Patent: US2014/128372, 2014, A1. Location in patent: Paragraph 0489; 0490; 0491; 0492; 0493
[3] Patent: US2017/57954, 2017, A1. Location in patent: Paragraph 0620; 0621; 0622; 0623; 0624
[4] Patent: US2016/347770, 2016, A1. Location in patent: Paragraph 0691; 0692; 0693; 0694; 0695
[5] Journal of Medicinal Chemistry, 1985, vol. 28, # 7, p. 876 - 892