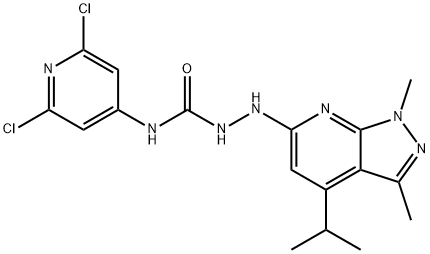
JTE-013
- CAS No.
- 383150-41-2
- Chemical Name:
- JTE-013
- Synonyms
- CS-2553;JTE-013 HCl;JTE 013 ,S7182;JTE-013;JTE 013;JTE013;JTE-013, 10 mM in DMSO;LPL Receptor,Apoptosis,JTE-013,Inhibitor,Lysophospholipid Receptor,JTE 013,inhibit,JTE013;1-(2,6-dichloro-4-pyridyl)-3-[(4-isopropyl-1,3-dimethyl-pyrazolo[3,4-b]pyridin-6-yl)amino]urea;1-(2,6-dichloropyridin-4-yl)-3-[(1,3-dimethyl-4-propan-2-ylpyrazolo[3,4-b]pyridin-6-yl)amino]urea;1-[1,3-Dimethyl-4-(2-methylethyl)-1H-pyrazolo[3,4-b]pyridin-6-yl]-4-(3,5-dichloro-4-pyridinyl)-semicarbazide;1-[1,3-Dimethyl-4-(2-methylethyl)-1H-pyrazolo[3,4-b]pyridin-6-yl]-4-(2,6-dichloro-4-pyridinyl)-semicarbazide
- CBNumber:
- CB52591408
- Molecular Formula:
- C17H19Cl2N7O
- Molecular Weight:
- 408.29
- MDL Number:
- MFCD09878275
- MOL File:
- 383150-41-2.mol
- MSDS File:
- SDS
| Product description | Number | Pack Size | Price |
| JTE-013 ≥98% (HPLC) | J4080 | 5mg | $151.05 |
| JTE-013 ≥98% (HPLC) | J4080 | 25mg | $441 |
| JTE-013 ≥98% | 10009458 | 1mg | $26 |
| JTE-013 ≥98% | 10009458 | 5mg | $98 |
| JTE-013 ≥98% | 10009458 | 10mg | $135 |
| More product size | |||
| Density | 1.50±0.1 g/cm3(Predicted) |
|---|---|
| storage temp. | 2-8°C |
| solubility | DMSO: ≥20mg/mL |
| pka | 9.32±0.43(Predicted) |
| form | powder |
| color | white to off-white |
| InChI | 1S/C17H19Cl2N7O/c1-8(2)11-7-14(22-16-15(11)9(3)25-26(16)4)23-24-17(27)20-10-5-12(18)21-13(19)6-10/h5-8H,1-4H3,(H,22,23)(H2,20,21,24,27) |
| InChIKey | RNSLRQNDXRSASX-UHFFFAOYSA-N |
| SMILES | Clc1nc(cc(c1)NC(=O)NNc2nc3[n](nc(c3c(c2)C(C)C)C)C)Cl |
| UNSPSC Code | 12352211 |
| NACRES | NA.77 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301 | |||||||||
| Precautionary statements | P301+P310 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 25 | |||||||||
| Safety Statements | 45 | |||||||||
| RIDADR | UN 2811 6.1 / PGIII | |||||||||
| WGK Germany | 3 | |||||||||
| Storage Class | 6.1C - Combustible acute toxic Cat.3 toxic compounds or compounds which causing chronic effects |
|||||||||
| Hazard Classifications | Acute Tox. 3 Oral | |||||||||
| NFPA 704 |
|
JTE-013 price More Price(21)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | J4080 | JTE-013 ≥98% (HPLC) | 383150-41-2 | 5mg | $151.05 | 2025-07-31 | Buy |
| Sigma-Aldrich | J4080 | JTE-013 ≥98% (HPLC) | 383150-41-2 | 25mg | $441 | 2025-07-31 | Buy |
| Cayman Chemical | 10009458 | JTE-013 ≥98% | 383150-41-2 | 1mg | $26 | 2024-03-01 | Buy |
| Cayman Chemical | 10009458 | JTE-013 ≥98% | 383150-41-2 | 5mg | $98 | 2024-03-01 | Buy |
| Cayman Chemical | 10009458 | JTE-013 ≥98% | 383150-41-2 | 10mg | $135 | 2024-03-01 | Buy |
JTE-013 Chemical Properties,Uses,Production
Description
Sphingosine-
Uses
JTE-013 has been used in in vitro blood?brain barrier (BBB) and blood?tumor barrier (BTB) assays.
Definition
ChEBI: 1-(2,6-dichloro-4-pyridinyl)-3-[(1,3-dimethyl-4-propan-2-yl-6-pyrazolo[3,4-b]pyridinyl)amino]urea is a pyrazolopyridine.
Biochem/physiol Actions
JTE-013 can enhance the excitability of small-diameter sensory neurons. It has the ability to repress sphingosine 1-phosphate (S1P)-mediated cellular migration.
storage
Store at +4°C
References
[1] MELISSA R PITMAN. The sphingosine 1-phosphate receptor 2/4 antagonist JTE-013 elicits off-target effects on sphingolipid metabolism.[J]. Scientific Reports, 2022: 454. DOI: 10.1038/s41598-021-04009-w
[2] MAKOTO OSADA. Enhancement of sphingosine 1-phosphate-induced migration of vascular endothelial cells and smooth muscle cells by an EDG-5 antagonist[J]. Biochemical and biophysical research communications, 2002, 299 3: Pages 483-487. DOI: 10.1016/s0006-291x(02)02671-2
[3] KAYO ARIKAWA. Ligand-dependent inhibition of B16 melanoma cell migration and invasion via endogenous S1P2 G protein-coupled receptor. Requirement of inhibition of cellular RAC activity.[J]. The Journal of Biological Chemistry, 2003, 278 35: 32841-32851. DOI: 10.1074/jbc.m305024200
[4] QIUMIN XU BMED . JTE-013 Alleviates Inflammatory Injury and Endothelial Dysfunction Induced by Sepsis In Vivo and In Vitro[J]. Journal of Surgical Research, 2021, 265: Pages 323-332. DOI: 10.1016/j.jss.2021.03.006
[5] MENGDIE WANG. Inhibition of sphingosine 1-phosphate (S1P) receptor 1/2/3 ameliorates biological dysfunction in rheumatoid arthritis fibroblast-like synoviocyte MH7A cells through Gαi/Gαs rebalancing[J]. Clinical and Experimental Pharmacology and Physiology, 2021, 48 8: 1080-1089. DOI: 10.1111/1440-1681.13460
[6] STEPHANIE S DUSABAN. Sphingosine 1-phosphate receptor 3 and RhoA signaling mediate inflammatory gene expression in astrocytes.[J]. Journal of Neuroinflammation, 2017: 111. DOI: 10.1186/s12974-017-0882-x
[7] NADINE AL ALAM Sawsan I K. FTY720P inhibits hepatic Na(+)-K(+) ATPase via S1PR2 and PGE2.[J]. Biochemistry and Cell Biology, 2016, 94 4: 371-377. DOI: 10.1139/bcb-2016-0025
JTE-013 Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 33024 | 60 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19552 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354; +17819995354 | marketing@targetmol.com | United States | 32470 | 58 |
| Alfa Chemistry | +1-5166625404; | Info@alfa-chemistry.com | United States | 20405 | 58 |
| InvivoChem | +1-708-310-1919 +1-13798911105 | sales@invivochem.cn | United States | 6391 | 58 |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | sales@molcore.com | China | 49734 | 58 |
| Wuhan Topule Biopharmaceutical Co., Ltd | +8618327326525 | masar@topule.com | China | 8467 | 58 |
| Shenyang Zhongshen Zekang Biomedical Technology Research Co., Ltd | +86-18341751992 +86-15382112998 | 757984502@qq.com | China | 892 | 58 |
| HANGZHOU LEAP CHEM CO., LTD. | +86-571-87711850 | market18@leapchem.com | China | 43339 | 58 |
| Aladdin Scientific | tp@aladdinsci.com | United States | 57505 | 58 |