マラリキシバット塩化物 化学特性,用途語,生産方法
効能
肝保護薬, ナトリウム胆汁酸共輸送体阻害薬
説明
Marilixibat chloride is an oral small-molecule ileal bile acid transporter (IBAT)
inhibitor developed by Mirum Pharmaceuticals and approved by
the USFDA for cholestatic pruritus in patients with Alagille
syndrome (ALGS). IBAT facilitates reabsorption of bile acids
from the ileum and is a key facilitator of bile acid enterohepatic
recirculation. Similar to surgical interruption of enterohepatic
recirculation, it was shown that inhibition of IBAT with 22
reduced pruritis in patients with ALGS.
合成
Gram-scale synthesis of marilixibat chloride. First, diethyl dibutylmalonate (22.1) was reduced with lithium aluminum hydride to the diol 22.2 in 95% yield. Next, the diol was converted to the bromoethanol (22.3) with HBr in acetic acid. Finally, the alcohol was oxidized to the bromoaldehyde (22.4) with sulfur trioxide pyridine complex.
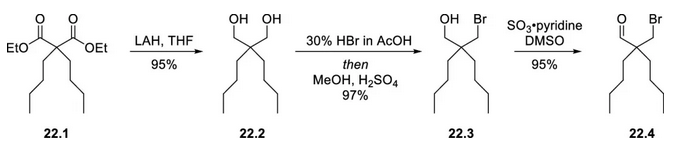
Next, 2-chloro-5-nitrobenzoic acid (22.5) was converted to the acid chloride with phosphorus pentachloride, and the resulting product was used directly in a Friedel-Crafts acylation with anisole to give the ketone 22.6. Deoxygenation of 22.6 was achieved with triethylsilane and trifluoromethanesulfonic acid in trifluoroacetic acid to give the aromatic hydrocarbon 22.7. Next, 22.7 was treated with sodium sulfide to displace the chlorine atom via a nucleophilic aromatic substitution reaction (SNAr); intermediate 22.4 (from Figure 6.4.2) was then added to the reaction to form the thioether via SN2 displacement of the bromide (22.4). The aldehyde group of the thioether was protected as a dimethyl acetal prior to oxidation of the thioether, which was oxidized using sodium acetate and peracetic acid in one reaction. Finally, the acetal was hydrolyzed and reduced to the aldehyde group to complete the three-step reaction in 97% overall yield to afford 22.8. Catalytic hydrogenation in the presence of formaldehyde and sulfuric acid converted the nitro group of 22.8 to a dimethylamino group to afford 22.9 in 89% yield. Treatment of 22.9 with potassium tert-butoxide in tetrahydrofuran (THF) resulted in a thermodynamically controlled stereoselective cyclization that set the hydroxyl and methoxyphenyl groups of 22.10 in a syn relationship. The cyclization product (22.10) was obtained in 93% yield and separated as a mixture of enantiomers in a 1:1 ratio by simulated moving bed (SMB) chromatography. Under conditions that racemize the non-target 4S,5S enantiomer using potassium tert-butoxide, it is recommended that it can be re-separated by SMB chromatography to improve the yield. The purified 4R,5R-22.10 was reacted with methionine and methanesulfonic acid to give phenol 22.11 in 80% yield.
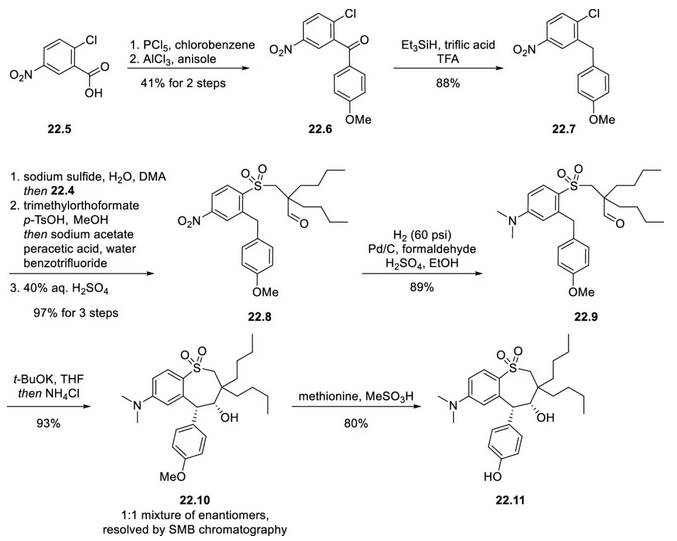
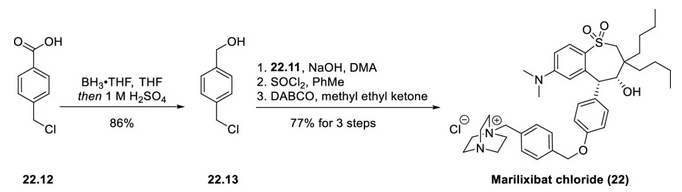
4-Chloromethylbenzoic acid was reduced to benzyl alcohol (22.13) by borane-THF adduct in 86% yield. Benzyl chloride 22.13 then reacted with phenol 22.11 in an SN2 reaction. The benzyl alcohol was then converted to the chloride, which was then displaced by diazabicyclo[2.2.2]octane (DABCO) to give Marilixibat Chloride (22) in 77% yield in 3 steps.
マラリキシバット塩化物 上流と下流の製品情報
原材料
準備製品